Vertebroplasty system
$0.00
Shipped From Abroad
Features:
- Minimally invasive surgery reduces tissue damage and bleeding;
- The operation is simple and quick, greatly shortening the operation time;
- Local anesthesia can be used to reduce surgical risks and postoperative complications;
- It can relieve pain immediately after surgery, effectively restore vertebral height, and correct spinal kyphosis.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
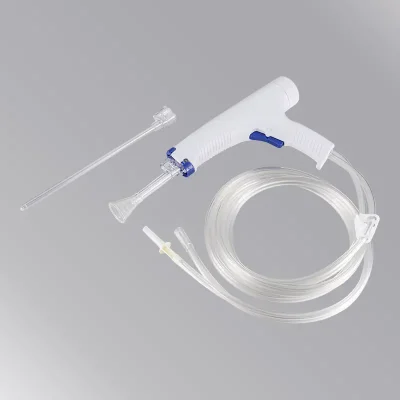
Vertebroplasty system components:
- Vertebral dilation balloon catheter: This device is mainly used to dilate the vertebral body to form a cavity during kyphoplasty surgery so that bone cement can be injected to restore and stabilize the vertebral body.
- Vertebroplasty Kit: This device is mainly used by medical institutions for percutaneous intervention of bones to establish working channels.
- Balloon dilation pressure pump: This device is mainly used to fill the balloon with liquid and pressurize it to achieve the effect of balloon dilation.
Vertebroplasty system configuration list (8G):
| Serial number | name | Specifications | quantity |
| 1 | Vertebral dilatation balloon catheter | KB0115/KB0210/KB0120 | 1 |
| 2 | Balloon dilatation pressure pump | BI-20A-10 | 1 |
| 3 | Vertebroplasty Kit | KT-00-01/KT-00-02/KT-00-03/KT-00-04 | 1 |
Vertebroplasty Kit Configuration List (8G):
| Serial number | name | Specifications | KT-00-01 | KT-00-02 | KT-00-03 | KT-00-04 |
| 1 | Puncture needle assembly | KT-01-01 | 2 | 2 | 1 | 1 |
| 2 | Puncture dilator assembly | KT-02-01 | 1 | / | 1 | / |
| 3 | Expander components | KT-03-01 | 2 | 2 | 1 | 1 |
| 4 | Bone Drill | KT-05-01 | 1 | 1 | 1 | 1 |
| 5 | Guide wire | KT-06-01 | 2 | 3 | 1 | 1 |
| 6 | Bone cement filler assembly | KT-04-01 | 6 | 6 | 3 | 3 |
Vertebroplasty system configuration list (11G):
| Serial number | name | Specifications | quantity |
| 1 | Vertebral dilatation balloon catheter | KB0115S/KB0210S/KB0120S | 1 |
| 2 | Balloon dilatation pressure pump | BI-20A-10 | 1 |
| 3 | Vertebroplasty Kit | KT-00-05/KT-00-06 | 1 |
Vertebroplasty Kit Configuration List (11G):
| Serial number | name | Specifications | KT-00-05 | KT-00-06 |
| 1 | Puncture dilator assembly | KT-07-01 | 2 | 1 |
| 2 | Bone cement filler assembly | KT-08-01 | 6 | 1 |
| 3 | Bone Drill | KT-09-01 | 1 | 1 |
Quick Comparison
| Vertebroplasty system remove | Disposable Surgical Lavage System (Internal battery type) remove | PICC/CVC Catheter Fixation Device remove | IV Start Kit remove | Suction Liner Type B remove | HR Lubricating Jelly OneShot Safe Wrap™ - STERILE remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Vertebroplasty system remove | Disposable Surgical Lavage System (Internal battery type) remove | PICC/CVC Catheter Fixation Device remove | IV Start Kit remove | Suction Liner Type B remove | HR Lubricating Jelly OneShot Safe Wrap™ - STERILE remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  | 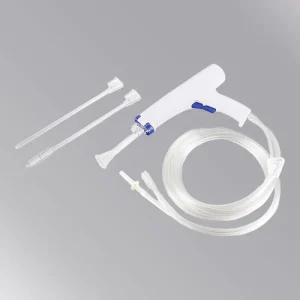 |  |  |  | 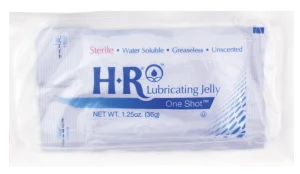 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130153-1 | SF1033560130115-2 | SF1033560130104-33 | SF1033560130104-32 | SF1033560130111-2 | SF1033560130149-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
Features:
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
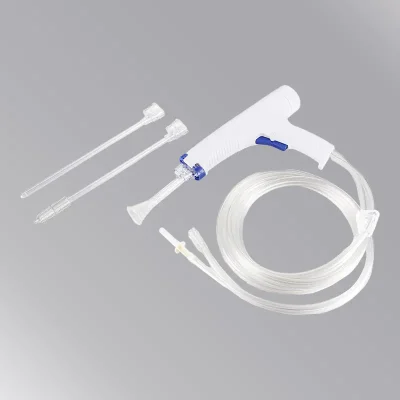
The Disposable Surgical Lavage System (Model WZ-WPL-01) is powered by 7 x 1.2v regular AA batteries and provides effective wound debridement following orthopedic procedures in trauma and joint arthroplasty.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
PICC catheter fixation device is composed of non-woven PU film, medical polymer coated medical pressure-sensitive adhesive and release material; used for the fixation of indwelling needle and the fixation of intravenous injection catheter or drainage tube.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
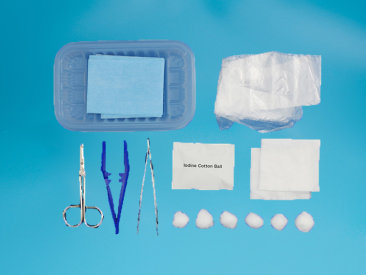
This product is mainly composed of transparent dressing A0607S, dry cotton swab, Povidone-Iodine Prep Swab, tourniquet, drape, infusion paste and paper-plastic packaging bag. (Can be customized according to clinical needs).
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
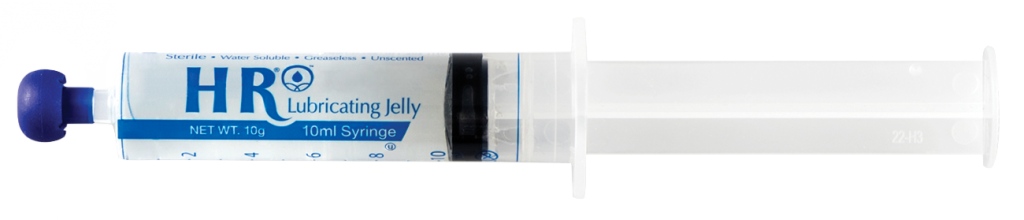
| Shipped From Abroad
HR’s SafeWrap™ packaging takes our OneShot offering and makes it operating room and sterile-field friendly. This product is our original HR Lubricating Jelly 1.25 oz.OneShot packaged in a STERILE SafeWrap™ sleeve.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content | Vertebroplasty system components:
Vertebroplasty system configuration list (8G):
| Product Description:
The Disposable Surgical Lavage System (Model WZ-WPL-01) is powered by 7 x 1.2v regular AA batteries and provides effective wound debridement following orthopedic procedures in trauma and joint arthroplasty.
Advantage:
1.Increase penetration between bone cement and osteotomy surface.
2.Reduce the incidence rate of fat embolism
3.Reduce the loosen rate of the implant,reduce reconstruction rate for artificial joint arthroplasty
4.Effective and rapidly cleansing of necrotic tissues,bacteria and foreign materials,minimizes risk of wound infection,accelerate wound healing.
5.Enlarge operation vision,shorten operation time.
6.Reduce the use of the dosage of antibiotics,save cost
7.Decrease the post-operative rate of adhesion.
Application:
| Product details PICC catheter fixation device is composed of non-woven PU film, medical polymer coated medical pressure-sensitive adhesive and release material; used for the fixation of indwelling needle and the fixation of intravenous injection catheter or drainage tube.Scope of use Used for fixation of PICC catheter, fixation of indwelling needle, fixation of central venous catheter or drainage tube and other suitable catheters.Instructions 1. Take out the product from the packaging bag after cleaning and sterilizing the puncture site. 2. Remove the release paper from the side of the slit to expose the glue surface. 3. Put the adhesive surface to the skin and smooth it from the middle to all around. 4. Fixed catheter.Precautions to take during the use of catheter fixing device 1. Single use. 2. Single use and Please don’t use once the pouch is torn. 3. Cannot instead of the suture, hemostasis, skin disinfection and other measures. 4. Cannot be used for the fixation of arterial duct.Product specification
| Product Description This product is mainly composed of transparent dressing A0607S, dry cotton swab, Povidone-Iodine Prep Swab, tourniquet, drape, infusion paste and paper-plastic packaging bag. (Can be customized according to clinical needs).Product Performance A. Disposable use to avoid iatrogenic infection; B. Sterile products; C. Integral sterilization, ready for use, safe and convenient for use; D. Compliance with the regulation of “strictly implement aseptic operation procedures, venous blood collection must be one person, one needle, one tube, one towel and one belt” as required by the Ministry of Health in Hospital Infection Management Regulations.Product Usage Applicable to intravenous infusion of indwelling needle in hospital and outpatient.Precautions 1. This product is sterilized by ethylene oxide and is safe and convenient to use; 2. The sterilization period of this product is limited to two years. If the package is damaged, it is forbidden to use; 3. For the production date and batch number, see the spray code; 4. This product is for single use, and it shall be destroyed in a centralized manner after use. Repeated use is strictly prohibited.Chuangqi Nursing Kit Products
| Description
B Type Model: YDB After the aspiration is complete, the bag automatic popup, very easy to lift with the lifting ring. Special design of the cover, made the cover more strength.And with more than 100ml tube liquid reflux extra space. All bags can be used individually or in a serial connection and accommodate all accessories for special clinical needs. #Suction bags without solidifying agent & filter are the basic type for general use. #Suction bags with solidifying agent are used to solidify the collection of the waste fluid. The waste fluid can be solidified, sterilized and deodorant immediately. #Suction bags with filter enable more hygiene operation as the valve prevents liquid spillage from the patient port before it is plugged.Specification: 2000ml |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information |









Reviews
There are no reviews yet.