DynaMesh-HIATUS
$0.00
Shipped From Abroad
DynaMesh®-HIATUS implants are intended for the surgical treatment of axial and/or paraoesophageal hiatal hernias, if a high risk of recurrence is expected with hiatoplasty using a simple suture technique. The devices permanently reinforce the soft tissue of the diaphragm in the region of the oesophageal hiatus.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
Defined Stability
Constriction of the mesh in the region of the hiatus may reduce the distance between the mesh implant and the oesophagus. DynaMesh®-HIATUS implants have rectangular pores and defined stability with a maximum elongation of only 8% [TR50, TR51].

Effective Porosity
All DynaMesh®-HIATUS implants have an effective porosity [TR51]. DynaMesh®-HIATUS implants are made of PVDF. PVDF has good biocompatibility, reducing the foreign body reaction compared to other materials such as polypropylene [1A, 2A, 4A, 68A, 100A, TR1].
DynaMesh®-HIATUS implants show little mesh shrinkage [51].

Smooth Warp-Knitted Selvedges
All DynaMesh®-HIATUS implants have smooth warp-knitted
selvedges.

Specifications
| Product | DynaMesh®-HIATUS |
| Surgical Treatment | Hiatal Hernias |
| Surgical Approach | Minimally Invasive / Open |
| Surgical Technique | Cruroplasty with mesh reinforcement |
| Fixation | Suture* / Tissue Adhesives / Tacks* |
| Defined Stability |
|
| Smooth Warp-Knitted Selvedges |
|
| Visible Technology |
|
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) |
| Polymer (Monofilament) | PVDF |
| Biocompatibility |
|
| Ageing Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR51] |
| * Traumatic fixation may only be used if injuries to the pericardium, aorta or vena cava can be ruled out with total certainty. |
Quick Comparison
| DynaMesh-HIATUS remove | McKesson Sprague Rappaport Stethoscope remove | Ambu Bag with Reservior remove | Oxygen Regulator (Japan Technology) remove | Portable Phelgm Suction Unit remove | Electric Suction Machine remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-HIATUS remove | McKesson Sprague Rappaport Stethoscope remove | Ambu Bag with Reservior remove | Oxygen Regulator (Japan Technology) remove | Portable Phelgm Suction Unit remove | Electric Suction Machine remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  |  | 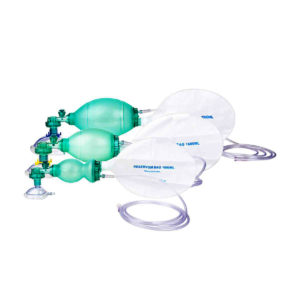 | 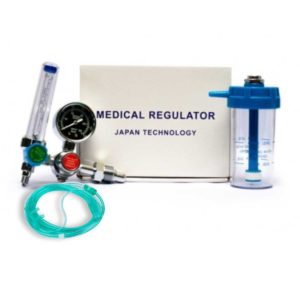 | 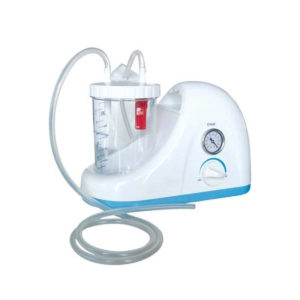 |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-7 | SF1033560084-35 | SF1033560084-41 | SF1033560084-48 | SF1033560084-10 | SF1033560084-16 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $17.00 | $17.00 | $17.00 | $54.50 | $152.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-HIATUS implants are intended for the surgical treatment of axial and/or paraoesophageal hiatal hernias, if a high risk of recurrence is expected with hiatoplasty using a simple suture technique. The devices permanently reinforce the soft tissue of the diaphragm in the region of the oesophageal hiatus.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In Stock
McKesson Sprague Rappaport Stethoscope: The McKesson Brand Two Sided Classic Nurse Stethoscope is an invaluable Diagnostic Instrument used by medical professionals when diagnosing heart, lung and vascular conditions. This high-quality stethoscope features an adjustable aluminum binaural, a single lumen 22-inch tube, a two-sided wide frequency chest piece, and comfortable ear tips.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
| In stock
| In Stock Portable Phelgm Suction Unit: Portable phlegm suction is designed based on developing orientation of similar products at home and abroad. It is a new generation of oil free lubrication suction device, suitable for use by the patient who has difficult in phlegm removal due to illness, coma and operations, as well as for aspiration liquid as pus and blood during clinical practice. It is commonly applied for using in the emergency room, operation room and for nursing and home health care. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
Power consumption: 120W
Maximum negative pressure: ≥0.09 MPa
Pump rate: ≥20 L/min
Noise: ≤60 dB
Continuous Working Time: 0-30 min
Bottle volume: 2500 ml, 2 pcs
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Defined Stability
Constriction of the mesh in the region of the hiatus may reduce the distance between the mesh implant and the oesophagus. DynaMesh®-HIATUS implants have rectangular pores and defined stability with a maximum elongation of only 8% [TR50, TR51].
Effective Porosity
All DynaMesh®-HIATUS implants have an effective porosity [TR51]. DynaMesh®-HIATUS implants are made of PVDF. PVDF has good biocompatibility, reducing the foreign body reaction compared to other materials such as polypropylene [1A, 2A, 4A, 68A, 100A, TR1].
DynaMesh®-HIATUS implants show little mesh shrinkage [51].

Smooth Warp-Knitted Selvedges
All DynaMesh®-HIATUS implants have smooth warp-knitted
selvedges.

Specifications
| McKesson Sprague Rappaport Stethoscope: The McKesson Brand Two Sided Classic Nurse Stethoscope is an invaluable Diagnostic Instrument used by medical professionals when diagnosing heart, lung and vascular conditions. This high-quality stethoscope features an adjustable aluminum binaural, a single lumen 22-inch tube, a two-sided wide frequency chest piece, and comfortable ear tips.
Features
|
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
Features:
|
| Portable Phelgm Suction Unit: Portable phlegm suction is designed based on developing orientation of similar products at home and abroad. It is a new generation of oil free lubrication suction device, suitable for use by the patient who has difficult in phlegm removal due to illness, coma and operations, as well as for aspiration liquid as pus and blood during clinical practice. It is commonly applied for using in the emergency room, operation room and for nursing and home health care.
Features:
| Specification :
Voltage: AC 220V, 50Hz or 110V, 60Hz
Power consumption: 120 W
Maximum negative pressure: ≥0.09 MPa
Negative pressure range: 0.02 MPa to Max negative pressure
Pump rate: ≥20 L/min
Noise: ≤60 dB
Continuous Working Time: 0-30 min
Rest Time≥30min
Bottle volume: 2500 ml, 2 pcs
Accessory: pedal switch (1pc), power switch(1pc),
filter(2 pcs), suction pipe(1pc, 2m in length, silica gel),
celiac suction pipe(1pc, 31cm in length, chrome copper)
Package: 1pc/carton
Box Size : 46.5*41.5*64.5cm
Net Weight: 11.5KGS
Gross Weight:13.8 KGS
Click Here To Download Catalogue | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||











Reviews
There are no reviews yet.