DynaMesh-PRP soft
$0.00
Shipped From Abroad
DynaMesh®-PRP soft and DynaMesh®-PRP visible implants are intended to be used as bridging material and reinforce soft tissue as part of surgical treatment for apical pelvic organ prolapse.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
Pectopexy
Bilateral Fixation on the Pectineal Ligament
Fig. left:
Apical mesh repair following hysterectomy with DynaMesh®-PRP soft / visible (03 cm x 15 cm)

Pectopexy after vaginal/cervical stump prolapse:
- Two implant sizes are available in the following dimensions DynaMesh®-PRP soft / visible 03 cm x 15 cm and DynaMesh®-PRP visible 03 cm x 18 cm.
- With greatly shortened vaginas, e.g., following a radical hysterectomy, DynaMesh®-PRP visible 03 cm x 18 cm can be used.

Pectopexy after vaginal/cervical stump prolapse with concomitant cystocele and/or rectocele: (pulsion cystocele / rectocele)
- Additional stabilisation of the anterior and/or posterior vaginal wall can be achieved with DynaMesh®-PRP visible 17 cm x 15 cm.

Pectopexy after uterine prolapse with uterine preservation:
- With a normal sized uterus, DynaMesh®-PRP visible 03 cm x 18 cm can be used and fixed in place on the posterior cervix.

Pectopexy after uterine prolapse with uterine preservation:
- With smaller uteri (below 100 g), anterior fixation of DynaMesh®-PRP soft / visible 03 cm x 15 cm can be selected as an alternative.
Specifications
| Product | DynaMesh®-PRP soft 03 cm x 15 cm (1) DynaMesh®-PRP visible 03 cm x 15 cm (2) DynaMesh®-PRP visible 03 cm x 18 cm (3) DynaMesh®-PRP visible 17 cm x 15 cm (4) |
| Surgical Treatment | Apical Pelvic Organ Prolapse (Uterus / Vaginal Stump / Cervical Stump) |
| Surgical Approach | Minimally Invasive / Open |
| Surgical Technique | Pectopexy (1) – (3) / Pectopexy with reinforcement of the vaginal walls in case of concomitant cystocele and/or rectocele.(4) |
| Fixation | – Pectineal ligament: non-absorbable suture – Vaginal stump: absorbable monofilament suture – Cervix: non-absorbable suture (size 0) |
| Smooth Warp-Knitted Selvedges | |
| Defined Elasticity | |
| Visible Technology | |
| Materials |
– Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) – (4)
– Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – (4) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) – (4) |
| Polymer (Monofilament) | PVDF |
| Biocompatibility | |
| Ageing Resistance | |
| Effective Porosity | |
| Klinge’s Mesh Classification | Class 1a [102P, TR121] |
Quick Comparison
| DynaMesh-PRP soft remove | Bistos BT- 720 Patient Monitor remove | Portable Phelgm Suction Unit remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | Spinal Epidural Pack remove | Bettermed BT667E Electric Operating Table remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-PRP soft remove | Bistos BT- 720 Patient Monitor remove | Portable Phelgm Suction Unit remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | Spinal Epidural Pack remove | Bettermed BT667E Electric Operating Table remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  |  | 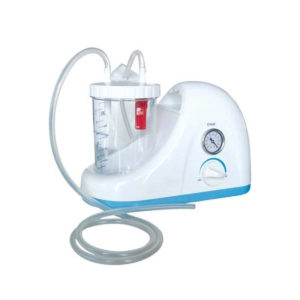 |  | 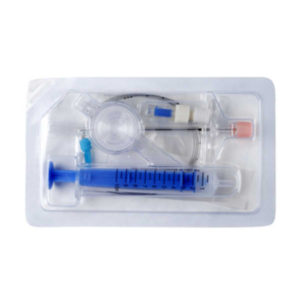 |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-10 | SF1033560059-8 | SF1033560084-10 | SF1033560084-37 | SF1033560084-25 | SF1033560084-50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $330.00 | $54.50 | $45.00 |
| $2,178.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-PRP soft and DynaMesh®-PRP visible implants are intended to be used as bridging material and reinforce soft tissue as part of surgical treatment for apical pelvic organ prolapse.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped from abroad Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer. Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock Portable Phelgm Suction Unit: Portable phlegm suction is designed based on developing orientation of similar products at home and abroad. It is a new generation of oil free lubrication suction device, suitable for use by the patient who has difficult in phlegm removal due to illness, coma and operations, as well as for aspiration liquid as pus and blood during clinical practice. It is commonly applied for using in the emergency room, operation room and for nursing and home health care. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
Omron M2 Upper Arm Blood Pressure Monitor Medium CufF: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage. zWith this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| In Stock
It is for performing spinal/epidural or combined spinal/epidural or never-block loco-regional anaesthesia on patinet in clinical surgery.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
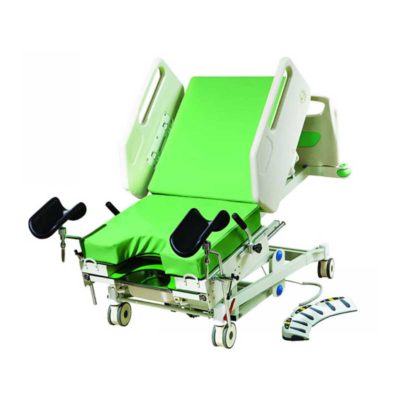
This operation table is used for the surgeries of chest ,abdomen ,ophthalmology,obstetrics,and urology.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Product | DynaMesh®-PRP soft 03 cm x 15 cm (1) DynaMesh®-PRP visible 03 cm x 15 cm (2) DynaMesh®-PRP visible 03 cm x 18 cm (3) DynaMesh®-PRP visible 17 cm x 15 cm (4) |
| Surgical Treatment | Apical Pelvic Organ Prolapse (Uterus / Vaginal Stump / Cervical Stump) |
| Surgical Approach | Minimally Invasive / Open |
| Surgical Technique | Pectopexy (1) – (3) / Pectopexy with reinforcement of the vaginal walls in case of concomitant cystocele and/or rectocele.(4) |
| Fixation | – Pectineal ligament: non-absorbable suture – Vaginal stump: absorbable monofilament suture – Cervix: non-absorbable suture (size 0) |
| Smooth Warp-Knitted Selvedges | |
| Defined Elasticity | |
| Visible Technology | |
| Materials |
– Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) – (4)
– Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – (4)
– Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) – (4) |
| Polymer (Monofilament) | PVDF |
| Biocompatibility | |
| Ageing Resistance | |
| Effective Porosity | |
| Klinge’s Mesh Classification | Class 1a [102P, TR121] |
- 3" color touch screen
- Standard Confirgurations: SpO2 and Pulse
- Optioal NIBP and Masimo SpO2
- Short/long trend graphic/trend table display
- Audio and visual alarm, adjustable alarm limit
- Automatic brightness adjustment
- Accurate SpO2 reading during motion or low perfusion
- Perfusion index data / bar graphic display
- Pitch tone variation for pulse rate
- Internal memory for data storage
- SD card for easy software upgrade
- Over 8 hours of operation with the re-chargeable li-ion battery
- PC software for data review and analysis on a computer
| Model | BT-720 | ||
|---|---|---|---|
| Display | 4.3" Color LCD Touch Screen | ||
| SpO2 | Measurement Range | 0-100% | |
| Accuracy (70-100%) | Adult/Pediatric | +/- 2% | |
| Neonate | +/- 3% | ||
| Accuracy (0-69%) | Unspecified | ||
| Perfusion Index | 0.05-20% | ||
| Pulse Rate | Range (bpm) | 25-250 | |
| NIBP* (Optional) | Method | Automatic Oscillometric | |
| Operation Mode | Manual/Automatic/STAT | ||
| Parameter | Systolic/Diastolic/Mean | ||
| Systolic Range (mmHg) | Adult | 40-270 | |
| Pediatric | 40-200 | ||
| Neonate | 40-130 | ||
| Diastolic Range | Adult | 100-210 | |
| Pediatric | 100-160 | ||
| Neonate | 10-90 | ||
| Mean Range | Adult | 20-230 | |
| Pediatric | 20-175 | ||
| Neonate | 20-100 | ||
| SpO2 Masimo* (Optional) | Masimo SpO2 | ||
| Battery | Type (Capacity) | Li-ion (4400mAh) | |
| Run time | 8 Hours | ||
| Charging Time | 8 Hours | ||
| Interface | RJ45, SD Card Slot | ||
| Warranty | 2 Years | ||
Click Here To Download Catalogue
- High negative pressure, large flux, low noise. It is a brand new mechanism design with suction bottle hidden inside, completely made of plastic. This design not only reduce the working noise but also make it more fashionable and precise.
- It adopts advanced overflow-protection design, which can prevent pus and mucus from leaking into the machine.
- Compact, lightweight and easy to carry.
| Brand Name | Stardent |
| Product Name | AC/DC portable suction |
| Model No | SD-H003A |
| Packing | Paper package 2pcs/ctn |
| Voltage | AC220/110V 50/60Hz DC:12V Electric current:4.5A |
| Battery capacity | 3500 mA |
| Max negative pressure value | ≥0.075MPa |
| Reservoir volume | 1000 mL |
| Power | 90VA |
| Pumping rate | 15L/min |
| Noise | ≤60dB(A) |
| G.W/N.W | 4.5/3KG |
Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage.With this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
- Fully automatic upper arm blood pressure monitor
- Intellisense™ technology
- Hypertension level indicator
- Irregular heartbeat detection
- Memory capacity for 21 readings with date and time stamp
- Cuff wrapping guide
- Clinically validated (International Protocol)
- Epidural needle 16G,
- Epidural catheter,
- Epidural filter 0.2 um,
- Catheter connector,
- LOR syringe









Reviews
There are no reviews yet.