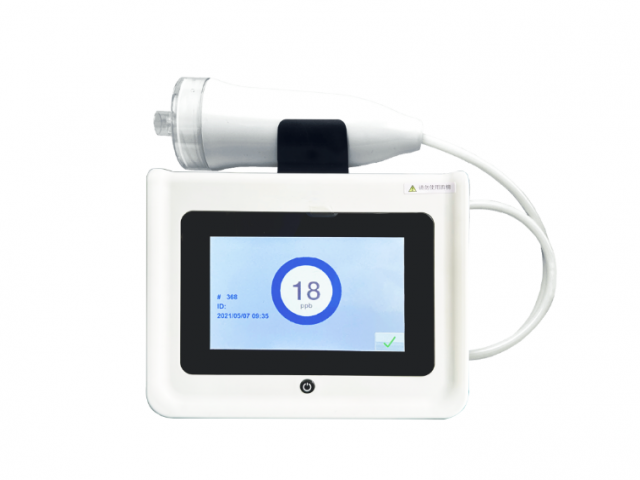
Exhaled Nitric Oxide Detector
$0.00
Shipped From Abroad
Exhaled nitric oxide detection is to measure the concentration of NO in human exhaled breath, referred to as FeNO detection
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
Exhaled Nitric Oxide Analyzer
The test can be performed with just 10 seconds of exhalation, and the test analysis time only takes 60 seconds.
For common diseases in departments such as respiratory medicine, pediatrics, otolaryngology, physical examination, and allergy, such as cough, asthma, chronic obstructive pulmonary disease, etc., during routine clinical examinations, it helps doctors to safely, quickly, and conveniently identify the type of inflammation, assess the level of inflammation, guide treatment, and monitor prognosis.
Detection principle:
Nitric oxide can be produced by a variety of intrinsic cells and inflammatory cells on the surface of the airways under the oxidation of nitric oxide synthase. Allergens and other stimuli induce airway inflammation, and cells such as eosinophils (EOS) release cytokines, activate nitric oxide synthase, and increase airway nitric oxide levels. Currently, FeNO detection can be used to identify chronic airway diseases with similar clinical manifestations such as COPD, asthma, and asthma -COPD overlap syndrome, and can be used to predict the treatment effect and prognosis of COPD patients. FeNO detection helps to improve the diagnosis and treatment of airway inflammatory diseases and can raise the clinical management level of airway inflammation to a new level. FeNO detection has the advantages of accurate results, reliable data, and good repeatability. It is an ideal non-invasive examination technology for airway inflammatory diseases.
Note:
1. Avoid eating, drinking, smoking, strenuous exercise, lung function, or other tests within 1 hour before sampling.
2. Avoid eating foods rich in nitrates or nitrites, such as broccoli, kale, lettuce, celery, radish, and smoked, pickled, or grilled foods within 3 hours.
3. Also ask and record the test time, medication, etc.
Quick Comparison
| Exhaled Nitric Oxide Detector remove | MIR Spirobank II Advance remove | M.I One ENT Treatment Chair (IC-3000) remove | Hersill V7 Plus AC High Vacuum Portable Suction Machine remove | Hersill V7 Plus B Emergency High Vacuum Portable Suction Machine remove | Flexible Endoscope remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Exhaled Nitric Oxide Detector remove | MIR Spirobank II Advance remove | M.I One ENT Treatment Chair (IC-3000) remove | Hersill V7 Plus AC High Vacuum Portable Suction Machine remove | Hersill V7 Plus B Emergency High Vacuum Portable Suction Machine remove | Flexible Endoscope remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image | 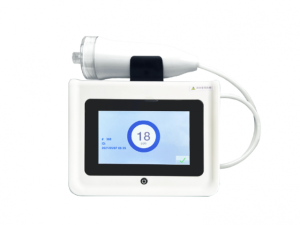 |  | 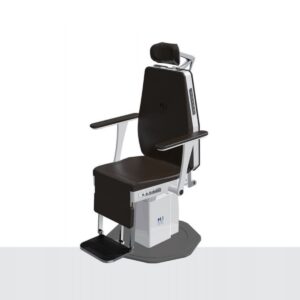 |  |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130159-6 | SF10335601291 | SF10335601262 | SF1033560450-5 | SF1033560450-6 | SF103356013089-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
Exhaled nitric oxide detection is to measure the concentration of NO in human exhaled breath, referred to as FeNO detection
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In Stock
The new Spirobank II Advanced from MIR is a user-friendly, intuitively operable mobile spirometer that has enough storage space to record up to 10,000 tests and enables immediate diagnosis thanks to the traffic light display.
Delivery & Availability:
Typically 7-14 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
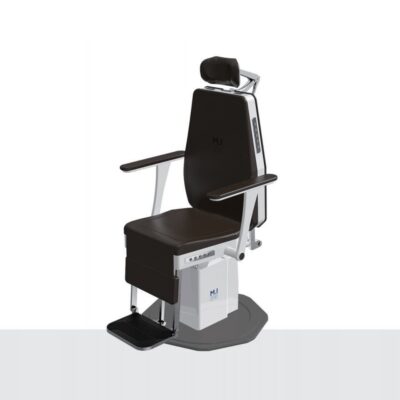
The IC-3000 ENT treatment chair features electrical height adjustment which is foot controlled, along with adjustable and removable armrests. Other features include 360° rotation (position lock), adjustable backrest from vertical to horizontal position, and an articulated headrest.
Delivery & Availability:
Typically 14-21 Working Days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped from Abroad The V7 range of high vacuum portable suction units are ideal for use in healthcare facilities (surgery, gynaecology, ENT), homes, ambulances, transportation and use in emergency situations. Delivery & Availability: Typically 21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from Abroad The V7 range of high vacuum portable suction units are ideal for use in healthcare facilities (surgery, gynaecology, ENT), homes, ambulances, transportation and use in emergency situations. Delivery & Availability: Typically 21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped From Abroad
Features
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content | Exhaled Nitric Oxide AnalyzerThe test can be performed with just 10 seconds of exhalation, and the test analysis time only takes 60 seconds. For common diseases in departments such as respiratory medicine, pediatrics, otolaryngology, physical examination, and allergy, such as cough, asthma, chronic obstructive pulmonary disease, etc., during routine clinical examinations, it helps doctors to safely, quickly, and conveniently identify the type of inflammation, assess the level of inflammation, guide treatment, and monitor prognosis.Detection principle:Nitric oxide can be produced by a variety of intrinsic cells and inflammatory cells on the surface of the airways under the oxidation of nitric oxide synthase. Allergens and other stimuli induce airway inflammation, and cells such as eosinophils (EOS) release cytokines, activate nitric oxide synthase, and increase airway nitric oxide levels. Currently, FeNO detection can be used to identify chronic airway diseases with similar clinical manifestations such as COPD, asthma, and asthma -COPD overlap syndrome, and can be used to predict the treatment effect and prognosis of COPD patients. FeNO detection helps to improve the diagnosis and treatment of airway inflammatory diseases and can raise the clinical management level of airway inflammation to a new level. FeNO detection has the advantages of accurate results, reliable data, and good repeatability. It is an ideal non-invasive examination technology for airway inflammatory diseases. Note: 1. Avoid eating, drinking, smoking, strenuous exercise, lung function, or other tests within 1 hour before sampling. 2. Avoid eating foods rich in nitrates or nitrites, such as broccoli, kale, lettuce, celery, radish, and smoked, pickled, or grilled foods within 3 hours. 3. Also ask and record the test time, medication, etc. |
The new Spirobank II Advanced from MIR is a user-friendly, intuitively operable mobile spirometer that has enough storage space to record up to 10,000 tests and enables immediate diagnosis thanks to the traffic light display. The real-time spirometry curve allows the results to be evaluated directly on the device.
MIR Spirobank II Advance Specifications
Click Here To Download Catalogue | The IC-3000 ENT treatment chair features electrical height adjustment which is foot controlled, along with adjustable and removable armrests. Other features include 360° rotation (position lock), adjustable backrest from vertical to horizontal position, and an articulated headrest. Features: - Convenient 5 position button Backrest Side Control Button Bottom Memory Button Backrest Rotation Button Backrest Control Button Armrest Turn Control Button - Electrical gear Driven Technical Specifications: Power Source: 220V AC, 50/60Hz Power Consumption: 400W Rotation: 3600 Electronic Brake system Up-Down Stroke: 300mm + 10%, Lifting Mechanism (Motor Support) Range of Back-Rest Movement: 0~900 Electronic Linear Actuator Headrest: Up/Down By Manual Dimension: W653xD881xH1278 Weight: Approx 120kg Arm-Rest: 0~900 by Manual Foot Switch: Up/Down/Front/Rear Remote Control: Hand Type, Up/Down/Front/Rear/Memory x 2Sit Position Memory x 4 | The V7 range of high vacuum portable suction units are ideal for use in healthcare facilities (surgery, gynaecology, ENT), homes, ambulances, transportation and use in emergency situations.
Features:
Click Here To Download Catalogue
| The V7 range of high vacuum portable suction units are ideal for use in healthcare facilities (surgery, gynaecology, ENT), homes, ambulances, transportation and use in emergency situations.
Features:
Click Here To Download Catalogue
| The flexible Naso-Pharyngo-Laryngoscope is manufactured in Germany to the highest of standards. It is highly suitable for both adult and paediatric use.
The high resolution, crystal clear optics ensure sharp image quality. The flexible tip can be moved 150° using the scope’s mechanism.
Technical Specifications
Total length
530 mm
Working length
300 mm
Flexible part diameter
3.4 mm or 2.8 mm
Angle deflection
up 150°, down 150°
Angle of vision
70°
Depth of focus
5-50 mm
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information |



















Reviews
There are no reviews yet.