DynaMesh-ENDOLAP
$0.00
Shipped From Abroad
DynaMesh-ENDOLAP and DynaMesh-ENDOLAP visible implants are intended for the surgical treatment of inguinal or femoral hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

Smooth Warp-Knitted Selvedges
The devices have smooth warp-knitted selvedges.

Choice of Method
The devices must be placed by means of laparoscopic transabdominal preperitoneal (TAPP) or endoscopic totally extraperitoneal (TEP)1) hernia repair. The colouring provides better intraoperative visibility and handling of the device.
1) Image of surgery courtesy of Dr. A. Kuthe,
DRK-Krankenhaus Clementinenhaus, Hanover, Germany
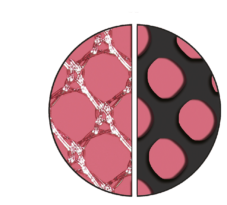
High Effective Porosity
The devices have a high effective porosity, which reduces inflammation and the risk of excessive scar formation. [103P, TR23]
Product Range
When selecting and cutting the device, sufficient defect overlap must be ensured to minimise the likelihood of the hernia recurring.
| DynaMesh®-ENDOLAP | 7.5 cm x 15 cm | PV100715F1/F3 |
| DynaMesh®-ENDOLAP | 10 cm x 15 cm | PV101015F1/F3/F10 |
| DynaMesh®-ENDOLAP | 12 cm x 15 cm | PV101215F1/F3/F10 |
| DynaMesh®-ENDOLAP | 13 cm x 15 cm | PV101315F1/F3 |
| DynaMesh®-ENDOLAP | 13 cm x 17 cm | PV101317F1/F3/F10 |
| DynaMesh®-ENDOLAP | 15 cm x 15 cm | PV101515F1/F3/F10 |
| DynaMesh®-ENDOLAP visible | 10 cm x 15 cm | PV141015F1/F3/F10 |
| DynaMesh®-ENDOLAP visible | 15 cm x 15 cm | PV141515F1/F3/F10 |
| DynaMesh®-ENDOLAP visible | 15 cm x 30 cm | PV141530F1 |
| FX = X unit(s)/box (e.g. F3 = 3 unit(s)/box) |
Specifications
| Product | DynaMesh®-ENDOLAP (1) DynaMesh®-ENDOLAP visible (2) |
| Surgical Treatment | Inguinal Hernias / Femoral Hernias |
| Surgical Approach | Laparoscopic / Endoscopic |
| Surgical Technique | TAPP / TEP |
| Mesh Position | Preperitoneal (Posterior) |
| Fixation | (None)* / Sutures / Tissue Adhesives / Tacks |
| Coloured Filaments | Green |
| Smooth Warp-Knitted Selvedges |
|
| Visible Technology |
|
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) (2) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) | PVDF |
| Biocompatibility |
|
| Ageing Resistance |
|
| Tear Propagation Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR23] |
| * Based on current knowledge, with the exception of large direct inguinal hernias (European Hernia Society classification: M3), it appears possible to dispense with any form of fixation. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
Quick Comparison
| DynaMesh-ENDOLAP remove | Bistos BT- 410 Medical Head Lamp remove | Bistos BT- 740 Patient Monitor remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | Oxygen Concentrator remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-ENDOLAP remove | Bistos BT- 410 Medical Head Lamp remove | Bistos BT- 740 Patient Monitor remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | Oxygen Concentrator remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image | 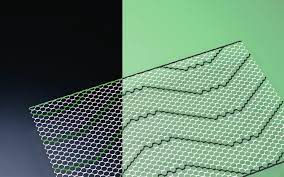 |  | 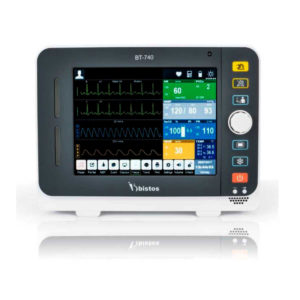 |  |  | 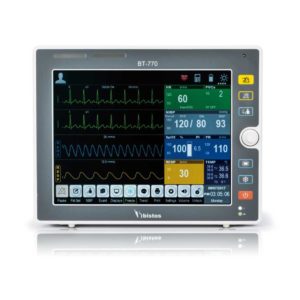 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-2 | SF1033560059-6 | SF1033560059-9 | SF1033560084-20 | SF1033560084-31 | SF1033560059-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $132.00 | $715.00 | $12.75 | $350.00 | $902.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh-ENDOLAP and DynaMesh-ENDOLAP visible implants are intended for the surgical treatment of inguinal or femoral hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
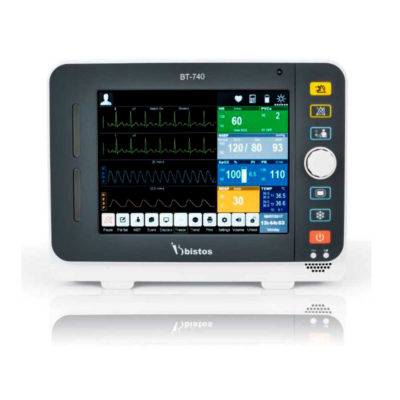
| Shipped from abroad Bistos BT- 410 Medical Head Lamp - Head-worn light BT-410 provides not only comfortable wear but also convenient for use during examinations or operations. - Ultra bright LED light with High density LED - Easy to adjust head strap & an angle - Extensive LED lifetime (more than 50,000 hours) - More than 4 hours of continuous use - An additional astral LED lamp (optional) - Attachable loupe (optional) Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from Abroad Bistos BT - 740 Patient Monitor: Bistos Patient Vital Signs Monitor, ECG, Resp., SpO2, NIBP, Temp., BT-740. The Bistos BT-740 is a multi-parameter patient monitor with an 8.4" TFT Color screen. The standard paramaters are ECG, respiratory, SpO2, NIBP and temperature. Additional options are EtCO2, IBP, Multi-gas, CO and Masimo SpO2. The BT-740 has a fanless design for quiet operation. Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
| In stock
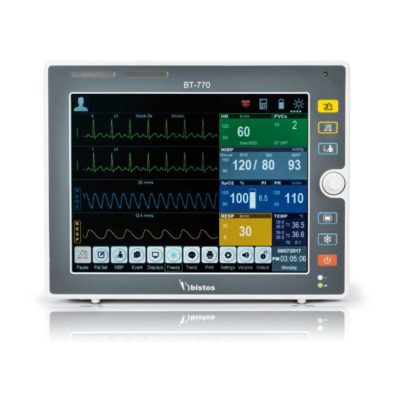
| Shipped from Abroad The Bistos BT-770 patient monitor is equipped with a 12.1" touchscreen display, which allows for an easy operation and readability with a powerful rechargeable battery guaranteeing a continuous operation of 5 hours to monitor ECG, SpO2, NIBP, temperature and respiration Delivery & Availability: Typically 14 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
1) Image of surgery courtesy of Dr. A. Kuthe, DRK-Krankenhaus Clementinenhaus, Hanover, Germany
High Effective Porosity The devices have a high effective porosity, which reduces inflammation and the risk of excessive scar formation. [103P, TR23] Product RangeWhen selecting and cutting the device, sufficient defect overlap must be ensured to minimise the likelihood of the hernia recurring.
Specifications
| Bistos BT- 410 Medical Head Lamp - Head-worn light BT-410 provides not only comfortable wear but also convenient for use during examinations or operations. - Ultra bright LED light with High density LED - Easy to adjust head strap & an angle - Extensive LED lifetime (more than 50,000 hours) - More than 4 hours of continuous use - An additional astral LED lamp (optional) - Attachable loupe (optional).
Features:
Illumination Uniformity:
Click Here To Download Catalogue | Bistos BT - 740 Patient Monitor: Bistos Patient Vital Signs Monitor, ECG, Resp., SpO2, NIBP, Temp., BT-740. The Bistos BT-740 is a multi-parameter patient monitor with an 8.4" TFT Color screen. The standard paramaters are ECG, respiratory, SpO2, NIBP and temperature. Additional options are EtCO2, IBP, Multi-gas, CO and Masimo SpO2. The BT-740 has a fanless design for quiet operation.
Features:
Click Here To Download Catalogue |
|
Bistos BT-770 is a 12.1" touchscreen patient monitor designed for easy operations.
SPECIFICATIONS
Click Here To Download Catalogue | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||








Reviews
There are no reviews yet.