DynaMesh-ENDOLAP 3D
$0.00
Shipped From Abroad
DynaMesh-ENDOLAP 3D and DynaMesh-ENDOLAP 3D visible implants are intended for the surgical treatment of inguinal or femoral hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

3D Shape
The device is a preformed three-dimensional mesh with a green central longitudinal marking, a green edge marking and centrally attached green filament ends.

Standardised Positioning
The device must be placed in such a way that the central green longitudinal marking is aligned with the inguinal ligament, the green central filament ends are located in projection to the junction of the inferior epigastric blood vessels to the external iliac blood vessels, and the green edge marking points dorsocaudally. The green filament ends must point to the user.
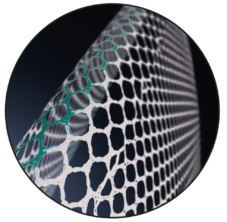
Varying Pore Size
The pore size of the device varies laterally to the longitudinal marking.
Product Range
When selecting and cutting the device, sufficient defect overlap must be ensured to minimise the likelihood of the hernia recurring.
| DynaMesh®-ENDOLAP 3D | 09 cm x 14 cm | PV130914F1/F3 |
| DynaMesh®-ENDOLAP 3D | 10 cm x 15 cm regular | PV131015F1/F3 |
| DynaMesh®-ENDOLAP 3D | 12 cm x 17 cm | PV131217F1/F5 |
| DynaMesh®-ENDOLAP 3D visible | 09 cm x 14 cm | PV120914F1/F3 |
| DynaMesh®-ENDOLAP 3D visible | 10 cm x 15 cm | PV121015F1/F3 |
| DynaMesh®-ENDOLAP 3D visible | 12 cm x 17 cm | PV121217F1 |
| FX = X unit(s)/box (e.g. F3 = 3 unit(s)/box) |
Can be used both for the right and the left side.
Features
| Product | DynaMesh®-ENDOLAP 3D (1) DynaMesh®-ENDOLAP 3D visible (2) |
| Surgical Treatment | Inguinal Hernias / Femoral Hernias |
| Surgical Approach | Laparoscopic / Endoscopic |
| Surgical Technique | TAPP / TEP |
| Mesh Position | Preperitoneal (Posterior) |
| Fixation | (None)* / Sutures / Tissue Adhesives / Tacks |
| Green Central and Edge Marking | |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) (2) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) |
PVDF
|
| Biocompatibility | |
| Ageing Resistance |
|
| Tear Propagation Resistance | |
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR23] |
| * Based on current knowledge, with the exception of large direct inguinal hernias (European Hernia Society classification: M3), it appears possible to dispense with any form of fixation. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#”(see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
Quick Comparison
| DynaMesh-ENDOLAP 3D remove | Bistos BT- 720 Patient Monitor remove | Ambu Bag with Reservior remove | McKesson Sprague Rappaport Stethoscope remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | De Lineb Oxygen Face Mask remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-ENDOLAP 3D remove | Bistos BT- 720 Patient Monitor remove | Ambu Bag with Reservior remove | McKesson Sprague Rappaport Stethoscope remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | De Lineb Oxygen Face Mask remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image | 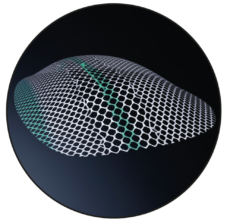 |  |  |  |  |  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560059-8 | SF1033560084-41 | SF1033560084-35 | SF1033560059-1 | SF1033560084-39 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $330.00 | $17.00 | $17.00 | $902.00 | $1.40 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh-ENDOLAP 3D and DynaMesh-ENDOLAP 3D visible implants are intended for the surgical treatment of inguinal or femoral hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped from abroad Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer. Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
| In Stock
McKesson Sprague Rappaport Stethoscope: The McKesson Brand Two Sided Classic Nurse Stethoscope is an invaluable Diagnostic Instrument used by medical professionals when diagnosing heart, lung and vascular conditions. This high-quality stethoscope features an adjustable aluminum binaural, a single lumen 22-inch tube, a two-sided wide frequency chest piece, and comfortable ear tips.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from Abroad The Bistos BT-770 patient monitor is equipped with a 12.1" touchscreen display, which allows for an easy operation and readability with a powerful rechargeable battery guaranteeing a continuous operation of 5 hours to monitor ECG, SpO2, NIBP, temperature and respiration Delivery & Availability: Typically 14 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
De Lineb Oxygen Face Mask: This mask provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask).
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Varying Pore Size The pore size of the device varies laterally to the longitudinal marking.
Features
| Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer.
Features:
Click Here To Download Catalogue |
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
Features:
| McKesson Sprague Rappaport Stethoscope: The McKesson Brand Two Sided Classic Nurse Stethoscope is an invaluable Diagnostic Instrument used by medical professionals when diagnosing heart, lung and vascular conditions. This high-quality stethoscope features an adjustable aluminum binaural, a single lumen 22-inch tube, a two-sided wide frequency chest piece, and comfortable ear tips.
Features
|
Bistos BT-770 is a 12.1" touchscreen patient monitor designed for easy operations.
SPECIFICATIONS
Click Here To Download Catalogue | De Lineb Oxygen Face Mask: This mask provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask).
Features:
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||









Reviews
There are no reviews yet.