DynaMesh-ENDOLAP 3D
$0.00
Shipped From Abroad
DynaMesh-ENDOLAP 3D and DynaMesh-ENDOLAP 3D visible implants are intended for the surgical treatment of inguinal or femoral hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

3D Shape
The device is a preformed three-dimensional mesh with a green central longitudinal marking, a green edge marking and centrally attached green filament ends.

Standardised Positioning
The device must be placed in such a way that the central green longitudinal marking is aligned with the inguinal ligament, the green central filament ends are located in projection to the junction of the inferior epigastric blood vessels to the external iliac blood vessels, and the green edge marking points dorsocaudally. The green filament ends must point to the user.
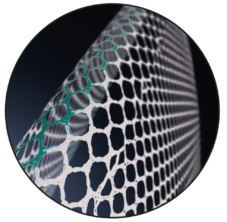
Varying Pore Size
The pore size of the device varies laterally to the longitudinal marking.
Product Range
When selecting and cutting the device, sufficient defect overlap must be ensured to minimise the likelihood of the hernia recurring.
| DynaMesh®-ENDOLAP 3D | 09 cm x 14 cm | PV130914F1/F3 |
| DynaMesh®-ENDOLAP 3D | 10 cm x 15 cm regular | PV131015F1/F3 |
| DynaMesh®-ENDOLAP 3D | 12 cm x 17 cm | PV131217F1/F5 |
| DynaMesh®-ENDOLAP 3D visible | 09 cm x 14 cm | PV120914F1/F3 |
| DynaMesh®-ENDOLAP 3D visible | 10 cm x 15 cm | PV121015F1/F3 |
| DynaMesh®-ENDOLAP 3D visible | 12 cm x 17 cm | PV121217F1 |
| FX = X unit(s)/box (e.g. F3 = 3 unit(s)/box) |
Can be used both for the right and the left side.
Features
| Product | DynaMesh®-ENDOLAP 3D (1) DynaMesh®-ENDOLAP 3D visible (2) |
| Surgical Treatment | Inguinal Hernias / Femoral Hernias |
| Surgical Approach | Laparoscopic / Endoscopic |
| Surgical Technique | TAPP / TEP |
| Mesh Position | Preperitoneal (Posterior) |
| Fixation | (None)* / Sutures / Tissue Adhesives / Tacks |
| Green Central and Edge Marking | |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) (2) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) |
PVDF
|
| Biocompatibility | |
| Ageing Resistance |
|
| Tear Propagation Resistance | |
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR23] |
| * Based on current knowledge, with the exception of large direct inguinal hernias (European Hernia Society classification: M3), it appears possible to dispense with any form of fixation. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#”(see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
Quick Comparison
| DynaMesh-ENDOLAP 3D remove | Bistos BT- 410 Medical Head Lamp remove | Bettermed BT667E Electric Operating Table remove | Portable Stainless Steel Autoclave Sterilizer remove | Oxygen Concentrator remove | 18Ltrs Bucket Autoclave remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-ENDOLAP 3D remove | Bistos BT- 410 Medical Head Lamp remove | Bettermed BT667E Electric Operating Table remove | Portable Stainless Steel Autoclave Sterilizer remove | Oxygen Concentrator remove | 18Ltrs Bucket Autoclave remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image | 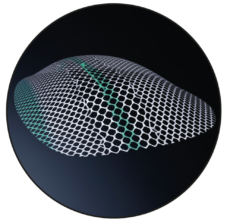 |  |  |  |  |  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560059-6 | SF1033560084-50 | SF1033560084-4 | SF1033560084-31 | SF1033560084-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $132.00 | $2,178.00 | $216.00 | $350.00 |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh-ENDOLAP 3D and DynaMesh-ENDOLAP 3D visible implants are intended for the surgical treatment of inguinal or femoral hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped from abroad Bistos BT- 410 Medical Head Lamp - Head-worn light BT-410 provides not only comfortable wear but also convenient for use during examinations or operations. - Ultra bright LED light with High density LED - Easy to adjust head strap & an angle - Extensive LED lifetime (more than 50,000 hours) - More than 4 hours of continuous use - An additional astral LED lamp (optional) - Attachable loupe (optional) Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
This operation table is used for the surgeries of chest ,abdomen ,ophthalmology,obstetrics,and urology.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
| In stock
| In Stock
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Varying Pore Size The pore size of the device varies laterally to the longitudinal marking.
Features
| Bistos BT- 410 Medical Head Lamp - Head-worn light BT-410 provides not only comfortable wear but also convenient for use during examinations or operations. - Ultra bright LED light with High density LED - Easy to adjust head strap & an angle - Extensive LED lifetime (more than 50,000 hours) - More than 4 hours of continuous use - An additional astral LED lamp (optional) - Attachable loupe (optional).
Features:
Illumination Uniformity:
Click Here To Download Catalogue | This operation table is used for the surgeries of chest, abdomen, ophthalmology, obstetricsand urology, etc.It has the following advantages:
●The board can shift longitudinally. It is made of Transparent material material.it can be X-rayed or scan when using along with C-ARM .
●The board is controlled by electric handspike to move up and down, lean from left to right and move back and forth.
●The leg board is dismantable. It is manual rotation and could be ooutreached with the force from the spring and gravity, convenient for urology surgery.
Technical Specification
| Features:
| Features:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information |







Reviews
There are no reviews yet.