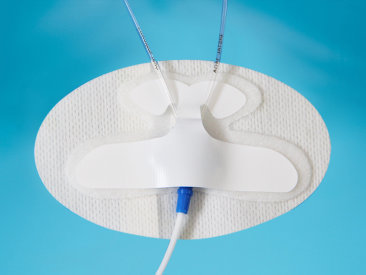
PICC/CVC Catheter Fixation Device
$0.00
Shipped From Abroad
PICC catheter fixation device is composed of non-woven PU film, medical polymer coated medical pressure-sensitive adhesive and release material; used for the fixation of indwelling needle and the fixation of intravenous injection catheter or drainage tube.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
Product details
PICC catheter fixation device is composed of non-woven PU film, medical polymer coated medical pressure-sensitive adhesive and release material; used for the fixation of indwelling needle and the fixation of intravenous injection catheter or drainage tube.
Scope of use
Used for fixation of PICC catheter, fixation of indwelling needle, fixation of central venous catheter or drainage tube and other suitable catheters.
Instructions
1. Take out the product from the packaging bag after cleaning and sterilizing the puncture site.
2. Remove the release paper from the side of the slit to expose the glue surface.
3. Put the adhesive surface to the skin and smooth it from the middle to all around.
4. Fixed catheter.
Precautions to take during the use of catheter fixing device
1. Single use.
2. Single use and Please don’t use once the pouch is torn.
3. Cannot instead of the suture, hemostasis, skin disinfection and other measures.
4. Cannot be used for the fixation of arterial duct.
Product specification
| Item Number | Product Usage | Specification |
|
A0207 |
Indwelling Needle Fixation Device |
2×7cm Non-foam cotton |
|
A0208 |
PICC/CVC Catheter Fixation Device |
2×8cm Peanut type |
|
A0309W |
Indwelling Needle Fixation Device |
3×9cm Non-foam cotton |
|
A0309WK |
Indwelling Needle Fixation Device |
3×9cm Blisterless ribbon holes |
|
A0309 |
PICC/CVC Catheter Fixation Device |
3×9cm Peanut type |
|
A0309F |
PICC/CVC Catheter Fixation Device |
3×9cm Aircraft type |
|
B0309 |
Indwelling Needle Fixation Device |
3×9cm Tear both sides |
|
B0607 |
Indwelling Needle Fixation Device |
6×7cm |
|
C0309 |
Urethral Catheter Fixation Device |
3×9cm Belt buckle type |
|
C0611 |
Urethral Catheter Fixation Device |
6×11cm Belt buckle type |
|
C0611F |
PICC/CVC Catheter Fixation Device |
6×11cm Aircraft type |
|
F0346 |
Fixed Band For Endotracheal Tube |
4.6cm L*3.2cm W |
|
F0609 |
Tracheal Tube Fixation Belt Accessories |
9×6-0.3cm Cotton foam |
|
F1 |
Endotracheal-Tube Fixation Device |
|
|
L0308 |
Nasal Feeding Tube Fixation Device |
3×8cm V Type |
|
L0710 |
Nasal Feeding Tube Fixation Device |
7×10cm L Type |
|
D0309 |
PICC/CVC Catheter Fixation Device |
3×9cm Statlock |
|
C0812 |
Urethral Catheter Fixation Device |
8×12cm Gourd type |
|
B0812 |
Drainage Tube Fixation Device |
8×12cm Gourd type |
|
F0556 |
Drainage Tube And Catheter Fixation |
4.8CM W*56Cm L |
Quick Comparison
| PICC/CVC Catheter Fixation Device remove | Dialysis Dressing Kit remove | HR Lubricating Jelly 3g OneShot remove | Suction Liner Type A remove | Disposable Surgical Lavage System (External battery type) remove | Disposable Medical Razor Blade remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | PICC/CVC Catheter Fixation Device remove | Dialysis Dressing Kit remove | HR Lubricating Jelly 3g OneShot remove | Suction Liner Type A remove | Disposable Surgical Lavage System (External battery type) remove | Disposable Medical Razor Blade remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  |  |  |  |  |  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130104-33 | SF1033560130104-30 | SF1033560130149-4 | SF1033560130111-1 | SF1033560130115-1 | SF1033560130102-147 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
PICC catheter fixation device is composed of non-woven PU film, medical polymer coated medical pressure-sensitive adhesive and release material; used for the fixation of indwelling needle and the fixation of intravenous injection catheter or drainage tube.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
This product is mainly composed of drape, tape, cotton roll, band-aid, povidone-iodine swab, latex gloves and tray. (Can be customized according to clinical needs).
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
HR Lubricating Jelly 3g OneShot is a sterile, single-use product designed to improve the application, minimize waste, and significantly reduce the risk of cross-contamination.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
Surgical Lavage Systems have become international standard irrigation device in orthopedic department(joint, trauma, spine), wound debridement and other surgical irrigation systems.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content | Product details PICC catheter fixation device is composed of non-woven PU film, medical polymer coated medical pressure-sensitive adhesive and release material; used for the fixation of indwelling needle and the fixation of intravenous injection catheter or drainage tube.Scope of use Used for fixation of PICC catheter, fixation of indwelling needle, fixation of central venous catheter or drainage tube and other suitable catheters.Instructions 1. Take out the product from the packaging bag after cleaning and sterilizing the puncture site. 2. Remove the release paper from the side of the slit to expose the glue surface. 3. Put the adhesive surface to the skin and smooth it from the middle to all around. 4. Fixed catheter.Precautions to take during the use of catheter fixing device 1. Single use. 2. Single use and Please don’t use once the pouch is torn. 3. Cannot instead of the suture, hemostasis, skin disinfection and other measures. 4. Cannot be used for the fixation of arterial duct.Product specification
| Product Description This product is mainly composed of drape, tape, cotton roll, band-aid, povidone-iodine swab, latex gloves and tray. (Can be customized according to clinical needs).Product Performance A. Disposable use to avoid iatrogenic infection; B. Sterile products; C. Integral sterilization, ready for use, safe and convenient for use; D. Compliance with the regulation of “strictly implement aseptic operation procedures, venous blood collection must be one person, one needle, one tube, one towel and one belt” as required by the Ministry of Health in Hospital Infection Management Regulations.Product Usage Postoperative daily care of dialysis patients in the hospital.Precautions 1. This product is sterilized by ethylene oxide and is safe and convenient to use; 2. The sterilization period of this product is limited to two years. If the package is damaged, it is forbidden to use; 3. For the production date and batch number, see the spray code; 4. This product is for single use, and it shall be destroyed in a centralized manner after use. Repeated use is strictly prohibited.Qichuang Nursing Kit Products
| Features:A Type
Model: YDA Bag with wide mouth design, large taper, and cover with a lifting ring, easy to take from the canister. Special filter design assure the stop line accurate,and with more than 50ml tube liquid reflux extra space, will not cause splash phenomenon. All bags can be used individually or in a serial connection and accommodate all accessories for special clinical needs. #Suction bags without solidifying agent & filter are the basic type for general use. #Suction bags with solidifying agent are used to solidify the collection of the waste fluid. The waste fluid can be solidified, sterilized and deodorant immediately. #Suction bags with filter enable more hygiene operation as the valve prevents liquid spillage from the patient port before it is plugged.Specification: 2000ml, 3500ml | Product Description
Surgical Lavage Systems have become international standard irrigation devices in orthop-edic department(joint, trauma, spine), wound debridement and other surgical irrigation since creation. The surgical lavage system is a sterilized device, it is designated to irrigate, wash, and evacuate debris from general soft tissue and surgical sites (e.g,, for soft-tissue wound or bone debridement, site cleaning, bone cement residue removal) with a pulsatile jet of sterile saline fluid. It is easy to be controlled and operated. The effective removal of foreign material, bacteria, tissue debris, hematoma from the wounds would be helpful to reduce the secondary injury around the normal tissue, reduce the risk of infection. As single use surgical lavage systems have the function of irrigation and negative pressure suction simultaneously, which can timely & effective clean tissue debris and polluted fluid, effectively prevent the spread of polution sources.
The systems have become essential in joint arthroplasty procedures, it is widely applyed in trauma orthopedic and surgical department. Especially in total hip and total knee arthroplasty procedures, surgical lavage systems are essential. As it can enhance the osmotic permeation between bone cement and osteotomy sclerotin, reduce the incidence rate of fat embolism caused by overflow of fat and sclerotin in the course of irrigation.
Advantage
| Features Blade: high quality single or double stainless steel blade, twain coated material, the blade is very sharp and durable. Main Function: • Shaving hairs off any length and density before operation. • Shaving hairs off any length and density daily for men and Women. Different colors available. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information |









Reviews
There are no reviews yet.