Catheter Fixation Device
$0.00
Shipped From Abroad
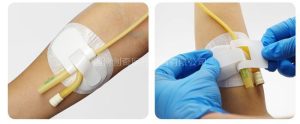
It is a long arc-shaped thin sheet with blunt ends and elasticity. There is a slight bulge in the center of the thin sheet in the opposite direction of the arc, and the inner wall has adhesive glue. The through-hole tube is fixed on the outside of the hole tube, and the base is fixed on the groin position of the inner thigh of the patient. The end is cross-fixed, thereby preventing the catheter from coming out of the urethra. The device can be easily removed from the catheter after use. The patient does not feel uncomfortable when using the device, and it can also be used to fix catheters in other parts of the body. It prevents infection caused by tube movement and greatly reduces the hospital infection rate.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
Product details
It is a long arc-shaped thin sheet with blunt ends and elasticity. There is a slight bulge in the center of the thin sheet in the opposite direction of the arc, and the inner wall has adhesive glue. The through-hole tube is fixed on the outside of the hole tube, and the base is fixed on the groin position of the inner thigh of the patient. The end is cross-fixed, thereby preventing the catheter from coming out of the urethra. The device can be easily removed from the catheter after use. The patient does not feel uncomfortable when using the device, and it can also be used to fix catheters in other parts of the body. It prevents infection caused by tube movement and greatly reduces the hospital infection rate.
Product performance
A. Single use to avoid nosocomial infection;
Precautions when using the catheter fixing device
1. Single use only.
2. Do not use if the packaging bag is damaged.
3. It cannot replace suturing, hemostasis, skin disinfection and other measures.
4. It cannot be used to fix arterial catheters.
Usage tips
Product Specifications
| Part Number | Product Usage | Specification |
| A0207 | Nasogastric feeding tube fixation device | 2×7cm without foam |
| A0208 | PICC catheter fixation device | 2×8cm Peanut Shape |
| A0309W | Indwelling needle fixation device | 3×9cm without foam |
| A0309WK | PICC catheter fixation device | 3×9cm non-foam with holes |
| A0309 | PICC catheter fixation device | 3×9cm Peanut Shape |
| A0309R | PICC catheter fixation device | 3×9cm Airplane type |
| A0309F | PICC catheter fixation device | 3×9cm Airplane type |
| B0309 | Epidural fixation device | 3×9cm tear on both sides |
| B0607 | Indwelling needle fixation device | 6×7cm |
| C0309 | Catheter Fixation Device | 3×9cm belt buckle type |
| C0611 | Catheter Fixation Device | 6×11cm belt buckle type |
| C0611F | Central venous catheter fixation device | 6×11cm Aircraft Type |
| F0346 | Endotracheal tube fixation belt | 4.6cm long*3.2cm wide |
| F0609 | Endotracheal tube fixing belt accessories | 9×6-0.3cm foam |
| F1 | Airway tube holder | |
| L0308 | Nasogastric feeding tube fixation device | 3×8cm V-shaped |
| L0710 | Nasogastric feeding tube fixation device | 7×10cm L-shaped |
| D0309 | PICC catheter fixation device | 3×9cm Silolock |
| C0812 | Catheter Fixation Device | 8×12cm gourd shape |
| B0812 | Drainage tube fixation device | 8×12cm gourd shape |
| F0556 | Drainage tube and urinary catheter fixation | 4.8CM wide*56Cm long |
Quick Comparison
| Settings | Catheter Fixation Device remove | HR Lubricating Jelly 2X OneShot remove | HR Lubricating Jelly 2X Flip-Top Tube remove | IV Start Kit remove | Disposable Surgical Lavage System (External battery type) remove | A0207 Catheter Fixation Device remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Catheter Fixation Device remove | HR Lubricating Jelly 2X OneShot remove | HR Lubricating Jelly 2X Flip-Top Tube remove | IV Start Kit remove | Disposable Surgical Lavage System (External battery type) remove | A0207 Catheter Fixation Device remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  | 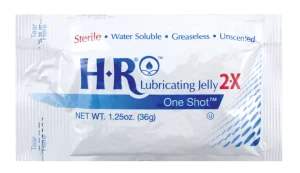 |  |  |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130104-24 | SF1033560130149-8 | SF1033560130149-3 | SF1033560130104-32 | SF1033560130115-1 | SF1033560130104-25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
It is a long arc-shaped thin sheet with blunt ends and elasticity. There is a slight bulge in the center of the thin sheet in the opposite direction of the arc, and the inner wall has adhesive glue. The through-hole tube is fixed on the outside of the hole tube, and the base is fixed on the groin position of the inner thigh of the patient. The end is cross-fixed, thereby preventing the catheter from coming out of the urethra. The device can be easily removed from the catheter after use. The patient does not feel uncomfortable when using the device, and it can also be used to fix catheters in other parts of the body. It prevents infection caused by tube movement and greatly reduces the hospital infection rate.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
HR Lubricating Jelly 2X OneShot is a sterile, single-use option with a thicker consistency than the original Lubricating Jelly formula. Our OneShot is designed to significantly reduce cross-contamination. The 2X formula is preferred by endoscopy physicians.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
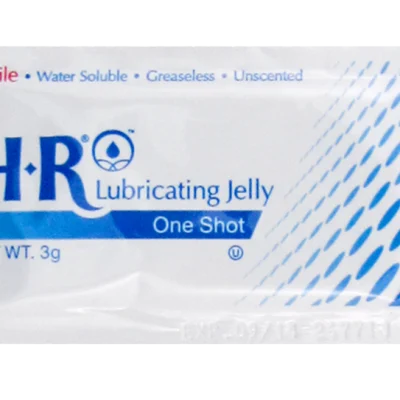
HR Lubricating Jelly 2X 2 oz. Flip-Top Tube is a sterile, multi-use option with a thicker consistency than the original Lubricating Jelly formula. The 2X formula is preferred by endoscopy physicians.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
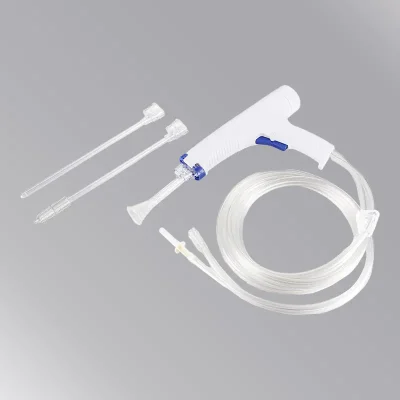
This product is mainly composed of transparent dressing A0607S, dry cotton swab, Povidone-Iodine Prep Swab, tourniquet, drape, infusion paste and paper-plastic packaging bag. (Can be customized according to clinical needs).
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
Surgical Lavage Systems have become international standard irrigation device in orthopedic department(joint, trauma, spine), wound debridement and other surgical irrigation systems.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped From Abroad
The catheter fixing device consists of a body surface fixing seat and a catheter fixing seat, which can be divided into adhesive type and strap type according to different body surface fixing methods. The body surface fixing seat is made of glue-coated non-woven fabric, polyurethane film and release paper or nylon burr. The catheter fixing seat is made of nylon burr or nylon burr and polypropylene plastic or nylon burr, polypropylene plastic and polyethylene foam, medical pressure-sensitive adhesive, and release paper.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content | Product details It is a long arc-shaped thin sheet with blunt ends and elasticity. There is a slight bulge in the center of the thin sheet in the opposite direction of the arc, and the inner wall has adhesive glue. The through-hole tube is fixed on the outside of the hole tube, and the base is fixed on the groin position of the inner thigh of the patient. The end is cross-fixed, thereby preventing the catheter from coming out of the urethra. The device can be easily removed from the catheter after use. The patient does not feel uncomfortable when using the device, and it can also be used to fix catheters in other parts of the body. It prevents infection caused by tube movement and greatly reduces the hospital infection rate.Product performance A. Single use to avoid nosocomial infection;Precautions when using the catheter fixing device 1. Single use only. 2. Do not use if the packaging bag is damaged. 3. It cannot replace suturing, hemostasis, skin disinfection and other measures. 4. It cannot be used to fix arterial catheters.Usage tips 
Product Specifications
|
|
| Product Description This product is mainly composed of transparent dressing A0607S, dry cotton swab, Povidone-Iodine Prep Swab, tourniquet, drape, infusion paste and paper-plastic packaging bag. (Can be customized according to clinical needs).Product Performance A. Disposable use to avoid iatrogenic infection; B. Sterile products; C. Integral sterilization, ready for use, safe and convenient for use; D. Compliance with the regulation of “strictly implement aseptic operation procedures, venous blood collection must be one person, one needle, one tube, one towel and one belt” as required by the Ministry of Health in Hospital Infection Management Regulations.Product Usage Applicable to intravenous infusion of indwelling needle in hospital and outpatient.Precautions 1. This product is sterilized by ethylene oxide and is safe and convenient to use; 2. The sterilization period of this product is limited to two years. If the package is damaged, it is forbidden to use; 3. For the production date and batch number, see the spray code; 4. This product is for single use, and it shall be destroyed in a centralized manner after use. Repeated use is strictly prohibited.Chuangqi Nursing Kit Products
| Product Description
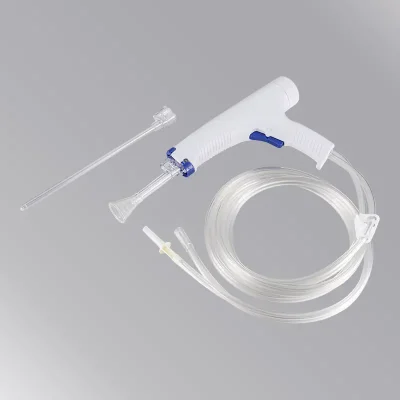
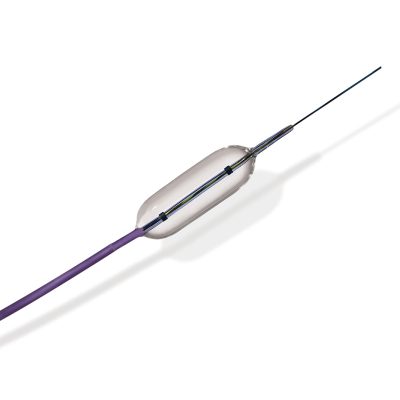
Surgical Lavage Systems have become international standard irrigation devices in orthop-edic department(joint, trauma, spine), wound debridement and other surgical irrigation since creation. The surgical lavage system is a sterilized device, it is designated to irrigate, wash, and evacuate debris from general soft tissue and surgical sites (e.g,, for soft-tissue wound or bone debridement, site cleaning, bone cement residue removal) with a pulsatile jet of sterile saline fluid. It is easy to be controlled and operated. The effective removal of foreign material, bacteria, tissue debris, hematoma from the wounds would be helpful to reduce the secondary injury around the normal tissue, reduce the risk of infection. As single use surgical lavage systems have the function of irrigation and negative pressure suction simultaneously, which can timely & effective clean tissue debris and polluted fluid, effectively prevent the spread of polution sources.
The systems have become essential in joint arthroplasty procedures, it is widely applyed in trauma orthopedic and surgical department. Especially in total hip and total knee arthroplasty procedures, surgical lavage systems are essential. As it can enhance the osmotic permeation between bone cement and osteotomy sclerotin, reduce the incidence rate of fat embolism caused by overflow of fat and sclerotin in the course of irrigation.
Advantage
| Product details The catheter fixing device consists of a body surface fixing seat and a catheter fixing seat, which can be divided into adhesive type and strap type according to different body surface fixing methods. The body surface fixing seat is made of glue-coated non-woven fabric, polyurethane film and release paper or nylon burr. The catheter fixing seat is made of nylon burr or nylon burr and polypropylene plastic or nylon burr, polypropylene plastic and polyethylene foam, medical pressure-sensitive adhesive, and release paper.Product performance The surface catheter fixation device is a disposable product.Precautions when using the catheter fixing device 1. Single use only. 2. Do not use if the packaging bag is damaged. 3. It cannot replace suturing, hemostasis, skin disinfection and other measures. 4. It cannot be used to fix arterial catheters.Scope of use Fixation of various surface cathetersProduct Specifications
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information |












Reviews
There are no reviews yet.