DynaMesh-CICAT
$0.00
Shipped From Abroad
DynaMesh-CICAT and DynaMesh-CICAT visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the prevention of incisional hernias, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

Anti-Slip Surface
The devices have an anti-slip surface that ensures stable positioning of the mesh with reduced fixation compared to other devices available on the market. [132PB, 133PB, 134]

Dynamometric Positioning
For the correct dynamometric positioning of the device, ensure that the green or black marking strips are in a cranio-caudal orientation.

High Effective Porosity
The devices have a high effective porosity, which is known to reduce inflammation and the risk of excessive scar formation. [103P, TR33, TR35]

The device must not be placed partially or completely intraperitoneally.
Specifications
| Product | DynaMesh®-CICAT (1) DynaMesh®-CICAT visible (2) |
| Surgical Treatment | Epigastric Hernias / Umbilical Hernias / Incisional Hernias Prevention: Incisional Hernia |
| Surgical Approach | Minimally Invasive / Open |
| Mesh Position | Extraperitoneal (onlay, sublay and/or preperitoneal) |
| Fixation | Sutures / Tacks / Tissue Adhesives |
| Marking Strips | Green (1) / Black (2) |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) |
PVDF
|
| Biocompatibility |
|
| Ageing Resistance |
|
| Dynamometric Properties |
|
| Tear Propagation Resistance |
|
| Anti-Slip Surface |
|
| Effective Porosity | |
| Klinge’s Mesh Classification | Class 1a [102P, TR33, TR35] |
| DynaMesh®-CICAT implants must be placed extraperitoneally in onlay, sublay and/or preperitoneal mesh position. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
Quick Comparison
| DynaMesh-CICAT remove | Elastomeric Pump (Disposable Infusion Pump) with PCA remove | Ambu Bag with Reservior remove | Portable Stainless Steel Autoclave Sterilizer remove | Ambu Oval Silicone resuscitator (Ambu Bag) remove | Portable Phelgm Suction Unit remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-CICAT remove | Elastomeric Pump (Disposable Infusion Pump) with PCA remove | Ambu Bag with Reservior remove | Portable Stainless Steel Autoclave Sterilizer remove | Ambu Oval Silicone resuscitator (Ambu Bag) remove | Portable Phelgm Suction Unit remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  | 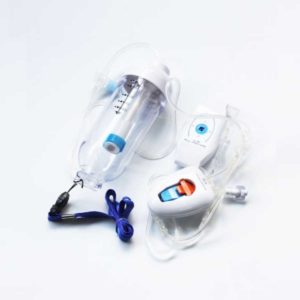 | 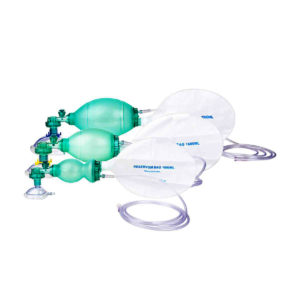 |  |  | 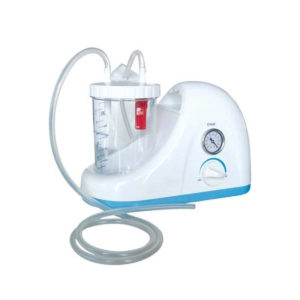 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-5 | SF1033560084-18 | SF1033560084-41 | SF1033560084-4 | SF1033560084-62 | SF1033560084-10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
|
| $17.00 | $216.00 | $27.00 | $54.50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | In stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | In stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh-CICAT and DynaMesh-CICAT visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the prevention of incisional hernias, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In Stock
This product make the tension of silicon capsule as the driving force to control the flow by micro porous tube, it was pre-selected by doctor base on patients'situation, and then and then handed over to the patients ' self- management “to relieve or ease the pain
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
| In Stock
| In Stock The Ambu Oval Silicone resuscitator is designed for manual ventilation of neonates though to adults. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock Portable Phelgm Suction Unit: Portable phlegm suction is designed based on developing orientation of similar products at home and abroad. It is a new generation of oil free lubrication suction device, suitable for use by the patient who has difficult in phlegm removal due to illness, coma and operations, as well as for aspiration liquid as pus and blood during clinical practice. It is commonly applied for using in the emergency room, operation room and for nursing and home health care. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Specifications
| This product make the tension of silicon capsule as the driving force to control the flow by micro porous tube, it was pre-selected by doctor base on patients'situation, and then and then handed over to the patients ' self- management “to relieve or ease the pain Product Components: one-way valve medicated device, elastic liquid storage device, liquid medicine filter, current limiting device, automatic liquid feeding device ( CBI & PCA ), pipe and the external connector; Product Features: 1. The continued to liquid (CBI) make the drug-stored sac mechanical stretch as a driving force, limited the flow through the micro porous tube to the patient safely, effectively, slightly, continuous uniform injection of liquid to analgesic effect; extrusion pump body does not affect the liquid normal velocity; liquid filtering device which can control the particles enter the body better; 2. Continued to liquid + self control to liquid ( CBI+PCA ) make the drug-stored sac mechanical stretch as a driving force, limited the flow through the micro porous tube to the patient safely, effectively, slightly, continuous uniform injection of liquid to analgesic effect; Automatic medicated function is the patients can medicate according to pain independently. Extrusion pump body does not affect the liquid normal velocity; liquid filtering device which can control the particles enter the body better; How to use: 1. Get analgesia pump out from sterile packaging, removing the cap of medicine inlet and outlet; pour the analgesic liquid in the liquid storage device, draw out the air from the liquid storage. 2. Finish medicating, waiting for the liquid outflow from the medicine outlet; please clamp tightly to stop flowing for preparation. 3. Control keys (PCA key) is a function key to add the medicine base on continue to medicated by patients' self-controlled. So the patients can self-control the PCA key under the guidance of doctors, according to the needs while they are in treatment. At the same time, doctor should fill in PCA treatment card, so that they can analyze the condition from PCA additional times. |
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
Features:
| Features:
| The Ambu Oval Silicone resuscitator is designed for manual ventilation of neonates though to adults.
Features
* International guidelines recommend that all manual resuscitators for infant and pediatric patients should be equipped with a pressure limitation mechanism so the airway pressure does not exceed 45 cm H2O. | Portable Phelgm Suction Unit: Portable phlegm suction is designed based on developing orientation of similar products at home and abroad. It is a new generation of oil free lubrication suction device, suitable for use by the patient who has difficult in phlegm removal due to illness, coma and operations, as well as for aspiration liquid as pus and blood during clinical practice. It is commonly applied for using in the emergency room, operation room and for nursing and home health care.
Features:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||








Reviews
There are no reviews yet.