DynaMesh-CICAT
$0.00
Shipped From Abroad
DynaMesh-CICAT and DynaMesh-CICAT visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the prevention of incisional hernias, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

Anti-Slip Surface
The devices have an anti-slip surface that ensures stable positioning of the mesh with reduced fixation compared to other devices available on the market. [132PB, 133PB, 134]

Dynamometric Positioning
For the correct dynamometric positioning of the device, ensure that the green or black marking strips are in a cranio-caudal orientation.

High Effective Porosity
The devices have a high effective porosity, which is known to reduce inflammation and the risk of excessive scar formation. [103P, TR33, TR35]

The device must not be placed partially or completely intraperitoneally.
Specifications
| Product | DynaMesh®-CICAT (1) DynaMesh®-CICAT visible (2) |
| Surgical Treatment | Epigastric Hernias / Umbilical Hernias / Incisional Hernias Prevention: Incisional Hernia |
| Surgical Approach | Minimally Invasive / Open |
| Mesh Position | Extraperitoneal (onlay, sublay and/or preperitoneal) |
| Fixation | Sutures / Tacks / Tissue Adhesives |
| Marking Strips | Green (1) / Black (2) |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) |
PVDF
|
| Biocompatibility |
|
| Ageing Resistance |
|
| Dynamometric Properties |
|
| Tear Propagation Resistance |
|
| Anti-Slip Surface |
|
| Effective Porosity | |
| Klinge’s Mesh Classification | Class 1a [102P, TR33, TR35] |
| DynaMesh®-CICAT implants must be placed extraperitoneally in onlay, sublay and/or preperitoneal mesh position. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
Quick Comparison
| DynaMesh-CICAT remove | Portable Phelgm Suction Unit remove | Littman Stethoscope remove | Portable Stainless Steel Autoclave Sterilizer remove | Oxygen Concentrator remove | Oxygen Concentrator 3Ltr. remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-CICAT remove | Portable Phelgm Suction Unit remove | Littman Stethoscope remove | Portable Stainless Steel Autoclave Sterilizer remove | Oxygen Concentrator remove | Oxygen Concentrator 3Ltr. remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  | 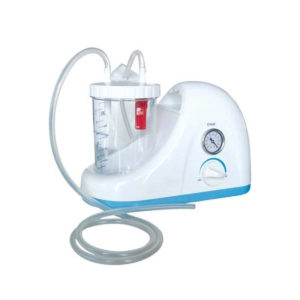 |  |  |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-5 | SF1033560084-10 | SF1033560084-5 | SF1033560084-4 | SF1033560084-31 | SF1033560084-40 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $54.50 | $13.20 | $216.00 | $350.00 | $273.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh-CICAT and DynaMesh-CICAT visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the prevention of incisional hernias, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
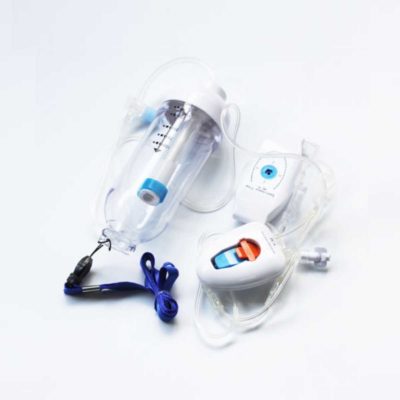
| In Stock Portable Phelgm Suction Unit: Portable phlegm suction is designed based on developing orientation of similar products at home and abroad. It is a new generation of oil free lubrication suction device, suitable for use by the patient who has difficult in phlegm removal due to illness, coma and operations, as well as for aspiration liquid as pus and blood during clinical practice. It is commonly applied for using in the emergency room, operation room and for nursing and home health care. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
| In stock
| In stock
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Specifications
| Portable Phelgm Suction Unit: Portable phlegm suction is designed based on developing orientation of similar products at home and abroad. It is a new generation of oil free lubrication suction device, suitable for use by the patient who has difficult in phlegm removal due to illness, coma and operations, as well as for aspiration liquid as pus and blood during clinical practice. It is commonly applied for using in the emergency room, operation room and for nursing and home health care.
Features:
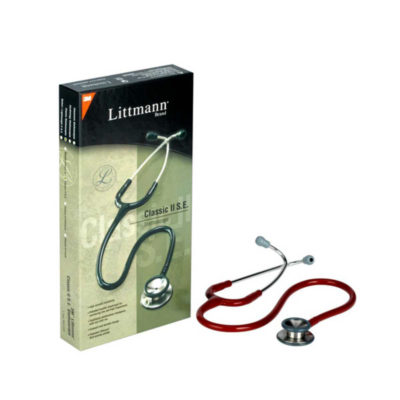
| Littman Stethoscope Features:
| Features:
| Our anesthesia system is a precision crafted, life-sustaining device. When moving to an O2 concentrator from tanks, you should expect the same attention to detail.
A full 8lpm O2 flowrate capability - gives better performance at lower flow rates
20psi pressure output - your anesthesia system was designed to work from pressure
O2 concentrations average 93% +/- 3%
Built-in Low O2 Concentration Alarm
Small footprint of 15"x15"x22"
Remarkably quiet
Save money over time vs. tanks
Eliminate contamination hazards of tanks coming and going in your facility
Works with ALL manufacturer's brands of anesthesia systems
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information |








Reviews
There are no reviews yet.