DynaMesh-CICAT
$0.00
Shipped From Abroad
DynaMesh-CICAT and DynaMesh-CICAT visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the prevention of incisional hernias, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

Anti-Slip Surface
The devices have an anti-slip surface that ensures stable positioning of the mesh with reduced fixation compared to other devices available on the market. [132PB, 133PB, 134]

Dynamometric Positioning
For the correct dynamometric positioning of the device, ensure that the green or black marking strips are in a cranio-caudal orientation.

High Effective Porosity
The devices have a high effective porosity, which is known to reduce inflammation and the risk of excessive scar formation. [103P, TR33, TR35]

The device must not be placed partially or completely intraperitoneally.
Specifications
| Product | DynaMesh®-CICAT (1) DynaMesh®-CICAT visible (2) |
| Surgical Treatment | Epigastric Hernias / Umbilical Hernias / Incisional Hernias Prevention: Incisional Hernia |
| Surgical Approach | Minimally Invasive / Open |
| Mesh Position | Extraperitoneal (onlay, sublay and/or preperitoneal) |
| Fixation | Sutures / Tacks / Tissue Adhesives |
| Marking Strips | Green (1) / Black (2) |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) |
PVDF
|
| Biocompatibility |
|
| Ageing Resistance |
|
| Dynamometric Properties |
|
| Tear Propagation Resistance |
|
| Anti-Slip Surface |
|
| Effective Porosity | |
| Klinge’s Mesh Classification | Class 1a [102P, TR33, TR35] |
| DynaMesh®-CICAT implants must be placed extraperitoneally in onlay, sublay and/or preperitoneal mesh position. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
Quick Comparison
| DynaMesh-CICAT remove | Littman Stethoscope remove | Oxygen Concentrator remove | Ambu Bag with Reservior remove | De Lineb Oxygen Face Mask remove | IBIS Neeo R9 Digital Surgical C-Arm remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-CICAT remove | Littman Stethoscope remove | Oxygen Concentrator remove | Ambu Bag with Reservior remove | De Lineb Oxygen Face Mask remove | IBIS Neeo R9 Digital Surgical C-Arm remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  |  |  | 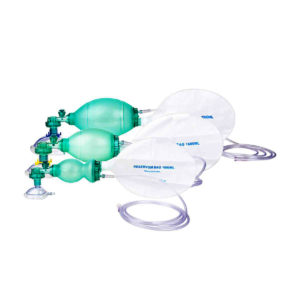 |  | 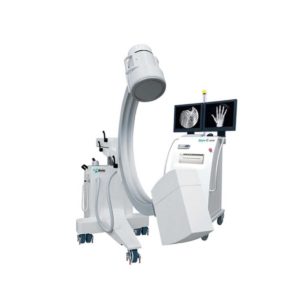 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-5 | SF1033560084-5 | SF1033560084-31 | SF1033560084-41 | SF1033560084-39 | SF1033560011-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $13.20 | $350.00 | $17.00 | $1.40 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh-CICAT and DynaMesh-CICAT visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the prevention of incisional hernias, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In Stock
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
| In stock
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
| In stock
De Lineb Oxygen Face Mask: This mask provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask).
| Shipped from Abroad Our Neeo “C” arms are easy to place, use and are specifically designed to be used in orthopedics, traumatology, abdominal surgery, urology, cardiology and operating rooms. Delivery & Availability: Typically 21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Specifications
| Littman Stethoscope Features:
|
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
Features:
| De Lineb Oxygen Face Mask: This mask provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask).
Features:
| Our Neeo “C” arms are easy to place, use and are specifically designed to be used in orthopedics, traumatology, abdominal surgery, urology, cardiology and operating rooms.
Using Neeo with the RTP (Real Time Processing) option it is possible to perform vascular, urological and cardiological diagnostics. One of the main functions, digital image subtraction, allows to see, as an example, the passage of contrast liquids in a tissue or in a venous or arterial duct; thanks to the possibility of looping, the acquired video can be reproduced several times to monitor more accurately the passage of the fluid within the area in question. Angiographic measurement is another useful function in the vascular field (QA Quantitative Angiography) that allows the measurement of stenoses. Finally, fluoroscopy allows the correct positioning of stents or expanders.
Neeo is used in various interventional and diagnostic procedures in traumatology and orthopedics wards and operating rooms as well. Thanks to low-dose fluoroscopy, it is possible to use the device for positioning bone or subcutaneous grafts, inserting K-wire (Kirschner wire) for stabilization of bone fragments or for the correct positioning of prostheses. The low dose emitted ensures safe use for both the patient and the surgeon or doctor on the operating field.
On the control panel there is a large touch screen display that allows to adjust the basic functions of the equipment. From this display it is possible to select and adjust the fluoroscopic data for the examination, activate or deactivate the laser pointer, select between pulsed, one shot or standard fluoroscopy, rotate the image and perform all operations on collimator. The four side buttons on the display offer the possibility to move the bow vertically thanks to an extremely silent motor.
Neeo has two 19 “medical grade monitors that can be positioned according to the needs of the medical practitioner. Work monitors and feedback monitors are separated to be managed independently. The possible movements are: rotation, revolution, tilting and possibility of height adjustment.
Features:
Click Here To Download Catalogue | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||









Reviews
There are no reviews yet.