DynaMesh-HIATUS
$0.00
Shipped From Abroad
DynaMesh®-HIATUS implants are intended for the surgical treatment of axial and/or paraoesophageal hiatal hernias, if a high risk of recurrence is expected with hiatoplasty using a simple suture technique. The devices permanently reinforce the soft tissue of the diaphragm in the region of the oesophageal hiatus.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
Defined Stability
Constriction of the mesh in the region of the hiatus may reduce the distance between the mesh implant and the oesophagus. DynaMesh®-HIATUS implants have rectangular pores and defined stability with a maximum elongation of only 8% [TR50, TR51].

Effective Porosity
All DynaMesh®-HIATUS implants have an effective porosity [TR51]. DynaMesh®-HIATUS implants are made of PVDF. PVDF has good biocompatibility, reducing the foreign body reaction compared to other materials such as polypropylene [1A, 2A, 4A, 68A, 100A, TR1].
DynaMesh®-HIATUS implants show little mesh shrinkage [51].

Smooth Warp-Knitted Selvedges
All DynaMesh®-HIATUS implants have smooth warp-knitted
selvedges.

Specifications
| Product | DynaMesh®-HIATUS |
| Surgical Treatment | Hiatal Hernias |
| Surgical Approach | Minimally Invasive / Open |
| Surgical Technique | Cruroplasty with mesh reinforcement |
| Fixation | Suture* / Tissue Adhesives / Tacks* |
| Defined Stability |
|
| Smooth Warp-Knitted Selvedges |
|
| Visible Technology |
|
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) |
| Polymer (Monofilament) | PVDF |
| Biocompatibility |
|
| Ageing Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR51] |
| * Traumatic fixation may only be used if injuries to the pericardium, aorta or vena cava can be ruled out with total certainty. |
Quick Comparison
| DynaMesh-HIATUS remove | IBIS Neeo R9 Digital Surgical C-Arm remove | Electric Suction Machine remove | Ambu Bag with Reservior remove | Littmann Classic III Stethoscope remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-HIATUS remove | IBIS Neeo R9 Digital Surgical C-Arm remove | Electric Suction Machine remove | Ambu Bag with Reservior remove | Littmann Classic III Stethoscope remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  | 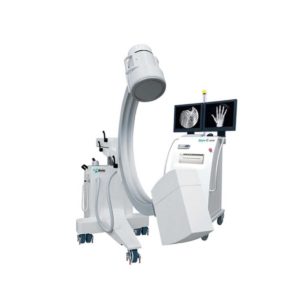 |  | 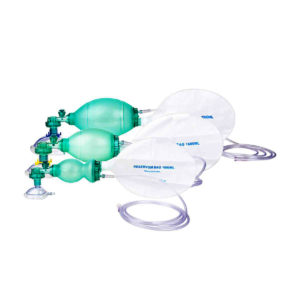 |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-7 | SF1033560011-1 | SF1033560084-16 | SF1033560084-41 | SF1033560084-34 | SF1033560084-20 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
|
| $152.00 | $17.00 | $34.00 | $12.75 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-HIATUS implants are intended for the surgical treatment of axial and/or paraoesophageal hiatal hernias, if a high risk of recurrence is expected with hiatoplasty using a simple suture technique. The devices permanently reinforce the soft tissue of the diaphragm in the region of the oesophageal hiatus.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped from Abroad Our Neeo “C” arms are easy to place, use and are specifically designed to be used in orthopedics, traumatology, abdominal surgery, urology, cardiology and operating rooms. Delivery & Availability: Typically 21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
Power consumption: 120W
Maximum negative pressure: ≥0.09 MPa
Pump rate: ≥20 L/min
Noise: ≤60 dB
Continuous Working Time: 0-30 min
Bottle volume: 2500 ml, 2 pcs
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
| In Stock
The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Defined Stability
Constriction of the mesh in the region of the hiatus may reduce the distance between the mesh implant and the oesophagus. DynaMesh®-HIATUS implants have rectangular pores and defined stability with a maximum elongation of only 8% [TR50, TR51].
Effective Porosity
All DynaMesh®-HIATUS implants have an effective porosity [TR51]. DynaMesh®-HIATUS implants are made of PVDF. PVDF has good biocompatibility, reducing the foreign body reaction compared to other materials such as polypropylene [1A, 2A, 4A, 68A, 100A, TR1].
DynaMesh®-HIATUS implants show little mesh shrinkage [51].

Smooth Warp-Knitted Selvedges
All DynaMesh®-HIATUS implants have smooth warp-knitted
selvedges.

Specifications
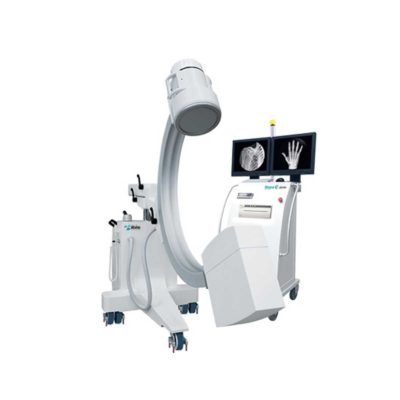
| Our Neeo “C” arms are easy to place, use and are specifically designed to be used in orthopedics, traumatology, abdominal surgery, urology, cardiology and operating rooms.
Using Neeo with the RTP (Real Time Processing) option it is possible to perform vascular, urological and cardiological diagnostics. One of the main functions, digital image subtraction, allows to see, as an example, the passage of contrast liquids in a tissue or in a venous or arterial duct; thanks to the possibility of looping, the acquired video can be reproduced several times to monitor more accurately the passage of the fluid within the area in question. Angiographic measurement is another useful function in the vascular field (QA Quantitative Angiography) that allows the measurement of stenoses. Finally, fluoroscopy allows the correct positioning of stents or expanders.
Neeo is used in various interventional and diagnostic procedures in traumatology and orthopedics wards and operating rooms as well. Thanks to low-dose fluoroscopy, it is possible to use the device for positioning bone or subcutaneous grafts, inserting K-wire (Kirschner wire) for stabilization of bone fragments or for the correct positioning of prostheses. The low dose emitted ensures safe use for both the patient and the surgeon or doctor on the operating field.
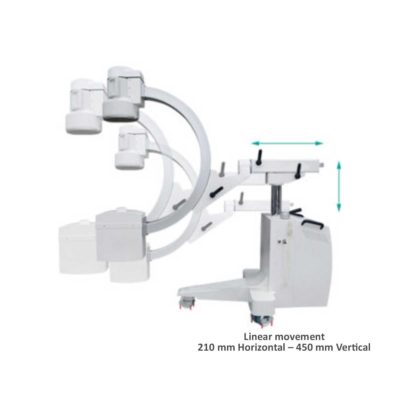
On the control panel there is a large touch screen display that allows to adjust the basic functions of the equipment. From this display it is possible to select and adjust the fluoroscopic data for the examination, activate or deactivate the laser pointer, select between pulsed, one shot or standard fluoroscopy, rotate the image and perform all operations on collimator. The four side buttons on the display offer the possibility to move the bow vertically thanks to an extremely silent motor.
Neeo has two 19 “medical grade monitors that can be positioned according to the needs of the medical practitioner. Work monitors and feedback monitors are separated to be managed independently. The possible movements are: rotation, revolution, tilting and possibility of height adjustment.
Features:
Click Here To Download Catalogue | Specification :
Voltage: AC 220V, 50Hz or 110V, 60Hz
Power consumption: 120 W
Maximum negative pressure: ≥0.09 MPa
Negative pressure range: 0.02 MPa to Max negative pressure
Pump rate: ≥20 L/min
Noise: ≤60 dB
Continuous Working Time: 0-30 min
Rest Time≥30min
Bottle volume: 2500 ml, 2 pcs
Accessory: pedal switch (1pc), power switch(1pc),
filter(2 pcs), suction pipe(1pc, 2m in length, silica gel),
celiac suction pipe(1pc, 31cm in length, chrome copper)
Package: 1pc/carton
Box Size : 46.5*41.5*64.5cm
Net Weight: 11.5KGS
Gross Weight:13.8 KGS
Click Here To Download Catalogue |
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
Features:
| The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
Full adult and pediatric auscultation is available through its dual head chestpiece simply by rotating the central spline to alternate between the two sides. The more compact one piece diaphragm and rim assemblies have further increased acoustic sensitivity. The binaurals have been redesigned with larger diameter ear tubes and a thicker single lumen binaural to transfer sound more effectively. The selector spline is now recessed to allow a flush fit with the binaural tube and now features a visual indicator for easy identification of the operational diaphragm.
Features
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||









Reviews
There are no reviews yet.