DynaMesh-HIATUS
$0.00
Shipped From Abroad
DynaMesh®-HIATUS implants are intended for the surgical treatment of axial and/or paraoesophageal hiatal hernias, if a high risk of recurrence is expected with hiatoplasty using a simple suture technique. The devices permanently reinforce the soft tissue of the diaphragm in the region of the oesophageal hiatus.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
Defined Stability
Constriction of the mesh in the region of the hiatus may reduce the distance between the mesh implant and the oesophagus. DynaMesh®-HIATUS implants have rectangular pores and defined stability with a maximum elongation of only 8% [TR50, TR51].

Effective Porosity
All DynaMesh®-HIATUS implants have an effective porosity [TR51]. DynaMesh®-HIATUS implants are made of PVDF. PVDF has good biocompatibility, reducing the foreign body reaction compared to other materials such as polypropylene [1A, 2A, 4A, 68A, 100A, TR1].
DynaMesh®-HIATUS implants show little mesh shrinkage [51].

Smooth Warp-Knitted Selvedges
All DynaMesh®-HIATUS implants have smooth warp-knitted
selvedges.

Specifications
| Product | DynaMesh®-HIATUS |
| Surgical Treatment | Hiatal Hernias |
| Surgical Approach | Minimally Invasive / Open |
| Surgical Technique | Cruroplasty with mesh reinforcement |
| Fixation | Suture* / Tissue Adhesives / Tacks* |
| Defined Stability |
|
| Smooth Warp-Knitted Selvedges |
|
| Visible Technology |
|
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) |
| Polymer (Monofilament) | PVDF |
| Biocompatibility |
|
| Ageing Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR51] |
| * Traumatic fixation may only be used if injuries to the pericardium, aorta or vena cava can be ruled out with total certainty. |
Quick Comparison
| DynaMesh-HIATUS remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | De Lineb Oxygen Face Mask remove | Ambu Oval Silicone resuscitator (Ambu Bag) remove | Oxygen Regulator (Japan Technology) remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-HIATUS remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | De Lineb Oxygen Face Mask remove | Ambu Oval Silicone resuscitator (Ambu Bag) remove | Oxygen Regulator (Japan Technology) remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | ||||||||||||||||||||||||||||||
| Image |  | 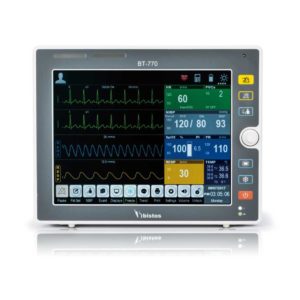 |  |  | 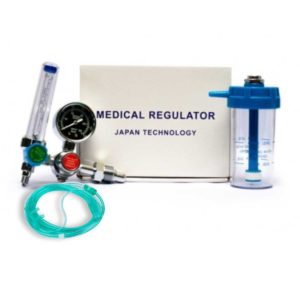 | 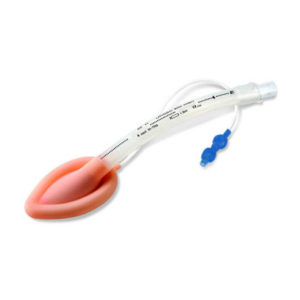 | ||||||||||||||||||||||||||||||
| SKU | SF1033560130161-7 | SF1033560059-1 | SF1033560084-39 | SF1033560084-62 | SF1033560084-48 | SF1033560084-20 | ||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||
| Price |
| $902.00 | $1.40 | $27.00 | $17.00 | $12.75 | ||||||||||||||||||||||||||||||
| Stock | In stock | |||||||||||||||||||||||||||||||||||
| Availability | In stock | |||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-HIATUS implants are intended for the surgical treatment of axial and/or paraoesophageal hiatal hernias, if a high risk of recurrence is expected with hiatoplasty using a simple suture technique. The devices permanently reinforce the soft tissue of the diaphragm in the region of the oesophageal hiatus.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
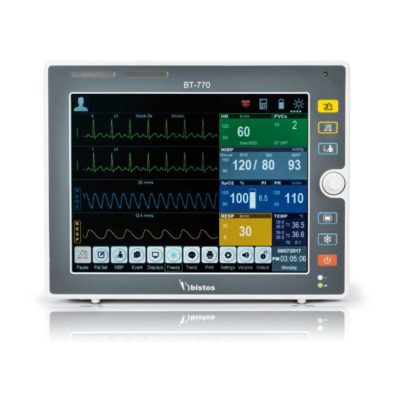
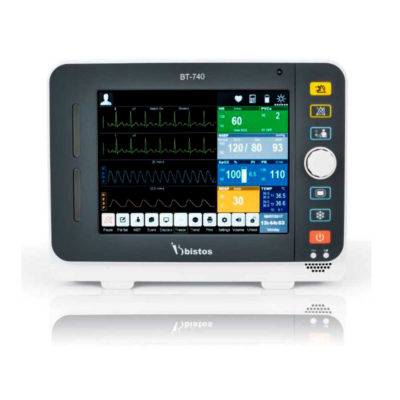
| Shipped from Abroad The Bistos BT-770 patient monitor is equipped with a 12.1" touchscreen display, which allows for an easy operation and readability with a powerful rechargeable battery guaranteeing a continuous operation of 5 hours to monitor ECG, SpO2, NIBP, temperature and respiration Delivery & Availability: Typically 14 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
De Lineb Oxygen Face Mask: This mask provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask).
| In Stock The Ambu Oval Silicone resuscitator is designed for manual ventilation of neonates though to adults. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
| In stock
| ||||||||||||||||||||||||||||||
| Content |
Defined Stability
Constriction of the mesh in the region of the hiatus may reduce the distance between the mesh implant and the oesophagus. DynaMesh®-HIATUS implants have rectangular pores and defined stability with a maximum elongation of only 8% [TR50, TR51].
Effective Porosity
All DynaMesh®-HIATUS implants have an effective porosity [TR51]. DynaMesh®-HIATUS implants are made of PVDF. PVDF has good biocompatibility, reducing the foreign body reaction compared to other materials such as polypropylene [1A, 2A, 4A, 68A, 100A, TR1].
DynaMesh®-HIATUS implants show little mesh shrinkage [51].

Smooth Warp-Knitted Selvedges
All DynaMesh®-HIATUS implants have smooth warp-knitted
selvedges.

Specifications
|
Bistos BT-770 is a 12.1" touchscreen patient monitor designed for easy operations.
SPECIFICATIONS
Click Here To Download Catalogue | De Lineb Oxygen Face Mask: This mask provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask).
Features:
| The Ambu Oval Silicone resuscitator is designed for manual ventilation of neonates though to adults.
Features
* International guidelines recommend that all manual resuscitators for infant and pediatric patients should be equipped with a pressure limitation mechanism so the airway pressure does not exceed 45 cm H2O. |
|
| ||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||
| Additional information |







Reviews
There are no reviews yet.