DynaMesh-LICHTENSTEIN
$0.00
Shipped From Abroad
DynaMesh-LICHTENSTEIN and DynaMesh-LICHTENSTEIN visible implants are intended for the surgical treatment of inguinal hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
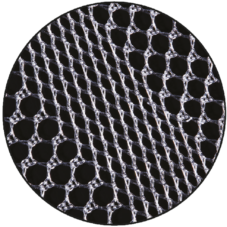
Varying Pore Size
The devices have areas with different pore sizes.
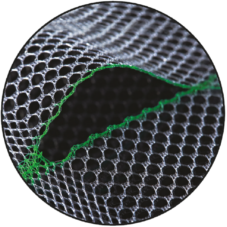
Prefabricated Slit & Smooth Warp-Knitted Selvedges
The devices have a prefabricated slit with smooth warp-knitted selvedges, and they have tear propagation resistance [TR82].
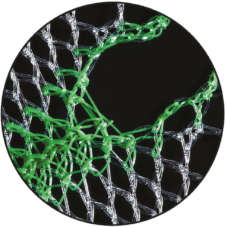
Visibility & Handling
The colouring provides better intraoperative visibility and handling of the device.
Product Range
When selecting the mesh size, ensure sufficient overlap!
| DynaMesh®-LICHTENSTEIN | 06 cm x 11 cm | PV110611F1/F3/F5/F10 |
| DynaMesh®-LICHTENSTEIN | 7.5 cm x 15 cm | PV110715F1/F3/F5/F10 |
| DynaMesh®-LICHTENSTEIN | 10 cm x 15 cm | PV111015F1/F3/F5 |
| DynaMesh®-LICHTENSTEIN visible | 06 cm x 11 cm | PV170611F3/F10 |
| DynaMesh®-LICHTENSTEIN visible | 7.5 cm x 15 cm | PV170715F1/F3/F10 |
| FX = X unit(s)/box (e.g. F3 = 3 unit(s)/box) |
It can be used both for the right and the left side.
Specifications
| Product | DynaMesh-LICHTENSTEIN (1) DynaMesh-LICHTENSTEIN visible (2) |
| Surgical Treatment | Inguinal Hernias |
| Surgical Approach | Open |
| Surgical Technique | Lichtenstein |
| Mesh Position | Onlay (Anterior) |
| Fixation | Sutures / Tacks / Tissue Adhesives* |
| Coloured Filaments | Green (1) / Black (2) |
| Smooth Warp-Knitted Selvedges | |
| Visible Technology |
|
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) | PVDF |
| Biocompatibility |
[TR1]
|
| Ageing Resistance |
[2A, 5VIT, 27A, 52VIT, 93A, 101]
|
| Tear Propagation Resistance |
[TR82]
|
| Effective Porosity |
High effective porosity reduces inflammation and the risk of excessive scar formation. [103P, TR83]
|
| Klinge’s Mesh Classification | Class 1a [102P, TR83] |
| * Tissue adhesive can be used for fixation for direct or indirect inguinal hernias with a defect size of 1.5 cm to 3 cm (European Hernia Society Classification: M2 or L2). |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
Quick Comparison
| DynaMesh-LICHTENSTEIN remove | Bistos BT- 720 Patient Monitor remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | McKesson Sprague Rappaport Stethoscope remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | Bistos BT- 410 Medical Head Lamp remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-LICHTENSTEIN remove | Bistos BT- 720 Patient Monitor remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | McKesson Sprague Rappaport Stethoscope remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | Bistos BT- 410 Medical Head Lamp remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  |  |  |  | 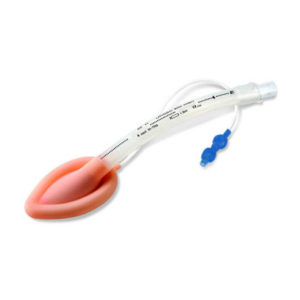 |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-1 | SF1033560059-8 | SF1033560084-37 | SF1033560084-35 | SF1033560084-20 | SF1033560059-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $330.00 | $45.00 | $17.00 | $12.75 | $132.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh-LICHTENSTEIN and DynaMesh-LICHTENSTEIN visible implants are intended for the surgical treatment of inguinal hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped from abroad Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer. Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
Omron M2 Upper Arm Blood Pressure Monitor Medium CufF: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage. zWith this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| In Stock
McKesson Sprague Rappaport Stethoscope: The McKesson Brand Two Sided Classic Nurse Stethoscope is an invaluable Diagnostic Instrument used by medical professionals when diagnosing heart, lung and vascular conditions. This high-quality stethoscope features an adjustable aluminum binaural, a single lumen 22-inch tube, a two-sided wide frequency chest piece, and comfortable ear tips.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
| Shipped from abroad Bistos BT- 410 Medical Head Lamp - Head-worn light BT-410 provides not only comfortable wear but also convenient for use during examinations or operations. - Ultra bright LED light with High density LED - Easy to adjust head strap & an angle - Extensive LED lifetime (more than 50,000 hours) - More than 4 hours of continuous use - An additional astral LED lamp (optional) - Attachable loupe (optional) Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Visibility & Handling The colouring provides better intraoperative visibility and handling of the device. Product RangeWhen selecting the mesh size, ensure sufficient overlap!
Specifications
| Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer.
Features:
Click Here To Download Catalogue | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage.With this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| McKesson Sprague Rappaport Stethoscope: The McKesson Brand Two Sided Classic Nurse Stethoscope is an invaluable Diagnostic Instrument used by medical professionals when diagnosing heart, lung and vascular conditions. This high-quality stethoscope features an adjustable aluminum binaural, a single lumen 22-inch tube, a two-sided wide frequency chest piece, and comfortable ear tips.
Features
|
| Bistos BT- 410 Medical Head Lamp - Head-worn light BT-410 provides not only comfortable wear but also convenient for use during examinations or operations. - Ultra bright LED light with High density LED - Easy to adjust head strap & an angle - Extensive LED lifetime (more than 50,000 hours) - More than 4 hours of continuous use - An additional astral LED lamp (optional) - Attachable loupe (optional).
Features:
Illumination Uniformity:
Click Here To Download Catalogue | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||







Reviews
There are no reviews yet.