DynaMesh-SIS direct
$0.00
Shipped From Abroad
DynaMesh®-SIS direct, DynaMesh®-SIS direct soft, and DynaMesh®-SIS direct visible implants are intended to be used as a mid-urethral sling for the surgical treatment of stress urinary incontinence caused by a hypermobile urethra and/or intrinsic sphincter deficiency. The devices permanently reinforce the soft tissue of the pelvic floor.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

Retropubic Tape Position
(Inside-Out)

Transobturator Tape Position
(Outside-In or Inside-Out)

DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible are positioned using the inside-out technique in case of a retropubic tape position, and using the outside-in or inside-out technique in case of a transobturator tape position.
The devices have a thread at the ends of the tape that is attached to the surgical instrument.
Several reusable instruments are available separately to position the device:

DynaMesh®-ISR01:
Instrument for transvaginal access for retropubic positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the inside-out technique.

Diameter: 5-7 cm
DynaMesh®-IST01 / DynaMesh®-IST02 / DynaMesh®-IST03:
Instrument set consisting of two instruments for transvaginal access for transobturator positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the inside-out or outside-in technique.

DynaMesh®-IVT01:
Instrument for transvaginal access for transobturator positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the outside-in technique.
Specifications
| Product | DynaMesh®-SIS (1) DynaMesh®-SIS soft (2) DynaMesh®-SIS visible (3) |
| Surgical Treatment | Stress Urinary Incontinence (SUI) |
| Surgical Approach | Transvaginal |
| Surgical Technique | TVT – Retropubic – Inside-Out / TOT – Transobturator – Outside-In |
| Fixation | None |
| Smooth Warp-Knitted Selvedges |
|
| Shape Stability |
|
| Defined Elasticity |
|
| Visible Technology |
|
| Materials |
– Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) – (3)
– Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) (2) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (3) |
| Polymer (Monofilament) |
PVDF
|
| Biocompatibility |
|
| Ageing Resistance |
|
| Effective Porosity | |
| Klinge’s Mesh Classification | Class 1a [102P, TR11] |
Quick Comparison
| DynaMesh-SIS direct remove | Portable Phelgm Suction Unit remove | Bistos BT- 720 Patient Monitor remove | Portable Stainless Steel Autoclave Sterilizer remove | Oxygen Regulator (Japan Technology) remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-SIS direct remove | Portable Phelgm Suction Unit remove | Bistos BT- 720 Patient Monitor remove | Portable Stainless Steel Autoclave Sterilizer remove | Oxygen Regulator (Japan Technology) remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image | 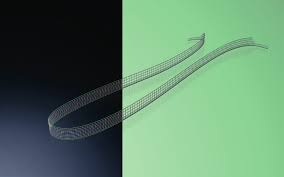 | 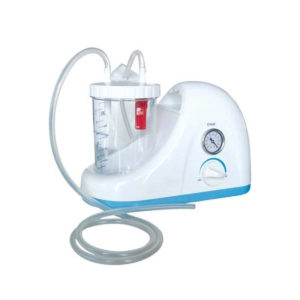 |  |  |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-8 | SF1033560084-10 | SF1033560059-8 | SF1033560084-4 | SF1033560084-48 | SF1033560084-37 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $54.50 | $330.00 | $216.00 | $17.00 | $45.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-SIS direct, DynaMesh®-SIS direct soft, and DynaMesh®-SIS direct visible implants are intended to be used as a mid-urethral sling for the surgical treatment of stress urinary incontinence caused by a hypermobile urethra and/or intrinsic sphincter deficiency. The devices permanently reinforce the soft tissue of the pelvic floor.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
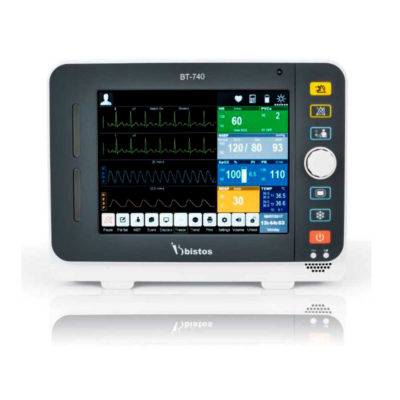
| In Stock Portable Phelgm Suction Unit: Portable phlegm suction is designed based on developing orientation of similar products at home and abroad. It is a new generation of oil free lubrication suction device, suitable for use by the patient who has difficult in phlegm removal due to illness, coma and operations, as well as for aspiration liquid as pus and blood during clinical practice. It is commonly applied for using in the emergency room, operation room and for nursing and home health care. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from abroad Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer. Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
| In stock
| In stock
Omron M2 Upper Arm Blood Pressure Monitor Medium CufF: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage. zWith this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Retropubic Tape Position
(Inside-Out)
Transobturator Tape Position
(Outside-In or Inside-Out)
DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible are positioned using the inside-out technique in case of a retropubic tape position, and using the outside-in or inside-out technique in case of a transobturator tape position.
The devices have a thread at the ends of the tape that is attached to the surgical instrument.
Several reusable instruments are available separately to position the device:
DynaMesh®-ISR01:
Instrument for transvaginal access for retropubic positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the inside-out technique.
DynaMesh®-IST01 / DynaMesh®-IST02 / DynaMesh®-IST03:
Instrument set consisting of two instruments for transvaginal access for transobturator positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the inside-out or outside-in technique.
DynaMesh®-IVT01: Instrument for transvaginal access for transobturator positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the outside-in technique. Specifications
| Portable Phelgm Suction Unit: Portable phlegm suction is designed based on developing orientation of similar products at home and abroad. It is a new generation of oil free lubrication suction device, suitable for use by the patient who has difficult in phlegm removal due to illness, coma and operations, as well as for aspiration liquid as pus and blood during clinical practice. It is commonly applied for using in the emergency room, operation room and for nursing and home health care.
Features:
| Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer.
Features:
Click Here To Download Catalogue | Features:
|
| Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage.With this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||







Reviews
There are no reviews yet.