MIRI® M Multiroom IVF Incubator
$0.00
Shipped From Abroad
The Esco Medical MIRI® M Multiroom IVF Incubator is a modular system with up to 18 independent and movable chambers. Each chamber is designed to maintain a stable and secure environment even during movement, ensuring optimal protection for samples at all times. Its recirculating gas system, with a VOC/HEPA filter and UVC light, maintains optimal conditions, while precise temperature control enhances sample safety. The display provides visual patient confirmation, and built-in software ensures digital verification and tracking, making it a reliable choice for modern IVF laboratories.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
From the Incubator to the Workstation – Stable Environment for the Best Outcome
Engineered for ultimate mobility and precision, the MIRI® M Chambers keep embryos in their ideal environment for extended periods, ensuring optimal conditions and more accurate results. Unlike conventional incubators that require frequent sample relocation, our innovative design preserves stability and integrity exactly where it’s needed. This advanced approach minimizes environmental disruptions for embryos, which supports better IVF outcomes
Features
Prolonged Undisturbed Incubation
The MIRI® M Chambers create an ideal environment for embryos, ensuring they develop optimally. Unlike traditional incubators that need constant sample movement, this innovative design minimizes temperature and gas fluctuations, maintaining stable culture conditions. As a result, enhanced accuracy, improved outcomes, and greater success rates in IVF procedures can be expected.
Maximized Capacity for Efficiency and Scalability
The MIRI® M Docking Station improves workflow by accommodating up to 18 separate chambers, ensuring efficient organization and easy access. Its scalable design allows labs to manage multiple samples simultaneously while optimizing the vital space inside the laboratory.
Safety and Control in Sample Transportation
Thoughtfully crafted with an ergonomic surface, MIRI® M Chambers offer a firm grip for enhanced control and stability. This innovative design minimizes handling risks, ensures sample integrity, and empowers users with the confidence they need for successful handling procedures.
Enhanced Safety with Built-in Mix-up Prevention
The MIRI® M Chambers guarantee accurate patient identification and traceability through a clear graphical display that prominently presents patient information. This built-in safeguard reduces errors, streamlines workflow, and enhances sample security throughout the treatment process. By continuously assigning each chamber to the patient, users can freely change the chamber’s position on the docking station without compromising traceability.
Advanced Alarm System
MIRI® M multiroom incubator provides information about alarm conditions through expansive pop-ups, icons, and colors on the Docking Station‘s and Chambers‘ screens, as well as backlights in the back panel.
Other Features
-
Improved Gas System: The superior gas system ensures rapid recovery and consistent performance using 100% CO2 and N2, automatically adjusting to connected chambers.
-
Quality Control Options: Each unit includes PT1000 temperature validation ports and gas sampling access for precise monitoring and peace of mind.
-
Gas Safety Assurance: A VOC/HEPA filter with UV sterilization ensures clean gas within MIRI® M Chamber. Conveniently placed on the side, the VOC/HEPA filter can be easily changed even in tight spaces
- Heating Optimization Plates: Specialized plates support a wide range of culture dishes, ensuring efficient heat transfer and precise temperature control.
General Specifications
MIRI® M Multiroom IVF Incubator Specifications
| Technical Specifications | MIRI® M Docking Station |
|---|---|
| Overall dimensions (W x D x H) | 806 × 670 × 643 mm (29.1 x 22.6 x 8.5”) |
| Weight | 85.6 kg (188.7 lbs) |
| Material | Mild steel / Aluminum / PET / Stainless steel |
| Power supply | 115-230VAC, 50-60Hz, 1.2 kW |
| Temperature Range | 35.0 – 39.0 °C |
| Temperature deviation from the setpoint | ± 0.1 °C |
| Gas Consumption (CO2) System stable (setpoint reached) – 6 chambers docked System stable (setpoint reached) – 18 chambers docked |
< 0.3 litre/hour < 0.5 litre/hour |
| Gas Consumption (N2) System stable (setpoint reached) – 6 chambers docked System stable (setpoint reached) – 18 chambers docked |
< 3.0 litre/hour < 5.0 litre/hour |
| CO2 Control Range | 3.0 – 12.0% |
| O2 Control Range | 3.0 – 10.0% |
| CO2 and O2 concentration deviation from the setpoint | ± 0.2% |
| CO2 Gas Pressure (input) | 0.7 – 1.5 bar (10.15 – 21.76 PSI) |
| N2 Gas Pressure (input) | 0.7 – 1.5 bar (10.15 – 21.76 PSI) |
| Operating altitude | Up to 2000 meters (6560 feet or 80kPa – 106kPa) |
| Shipping weight | 122.3 kg (269.6 lbs) (including the pallet’s weight) |
| Shipping dimension | 920 x 800 x 850 mm (36.2 x 31.5 x 33.5″) (device on the pallet) |
MIRI® M Chamber’s Specifications
| Technical Specifications | MIRI® M Chamber |
|---|---|
| Overall dimensions (W x D x H) | 113 x 215 x 59 mm (4.5 x 8.5 x 2.3”) |
| Net Weight | 1.03 kg (2.2 lbs) |
| Material | Aluminum / PET / Stainless steel |
| Rated power input | 24V DC, 1.58A, 38W |
| Battery | 3.6V, 12.42 Wh, Li-Ion |
| Undocked chamber battery time | 30 minutes (without the lid opening) |
| Shipping weight | 1.3 kg (2.9 lbs) |
| Shipping dimension | 122 x 324 x 163 mm (4.8 x 12.8 x 6.4″) (when sent separate) |
Quick Comparison
| MIRI® M Multiroom IVF Incubator remove | Nikon SMZ745 Stereoscopic Microscope remove | Skyla HB1 POC Clinical Chemistry Auto Analyzer remove | 8 Bucket Centrifuge remove | Little Sister Table Top Autoclave remove | Automatic Pipette remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | MIRI® M Multiroom IVF Incubator remove | Nikon SMZ745 Stereoscopic Microscope remove | Skyla HB1 POC Clinical Chemistry Auto Analyzer remove | 8 Bucket Centrifuge remove | Little Sister Table Top Autoclave remove | Automatic Pipette remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  | 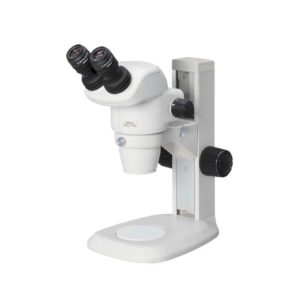 |  |  |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560084-49 | SF1033560060-1 | SF1033560084-114 | SF1033560084-46 | SF1033560084-130 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
|
|
| $72.50 | $1,350.00 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
The Esco Medical MIRI® M Multiroom IVF Incubator is a modular system with up to 18 independent and movable chambers. Each chamber is designed to maintain a stable and secure environment even during movement, ensuring optimal protection for samples at all times. Its recirculating gas system, with a VOC/HEPA filter and UVC light, maintains optimal conditions, while precise temperature control enhances sample safety. The display provides visual patient confirmation, and built-in software ensures digital verification and tracking, making it a reliable choice for modern IVF laboratories.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
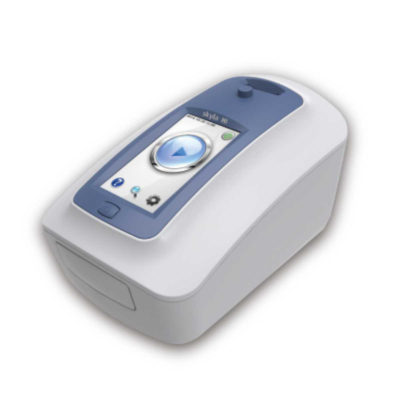
| In stock Nikon SMZ745 Stereoscopic Microscope: Featuring a zoom magnification of 7.5X and a long working distance of 115mm, Nikon SMZ745 microscopes are well suited for both industrial and biomedical applications. The adoption of a new total reflection prism produces brighter images with higher contrast, and the anti-mold design allows the microscope to be used in environments where the temperature and humidity are high. The SMZ-745T trinocular model comes equipped with a microscope camera port and a built-in 0.55x c-mount adapter, permitting direct mounting of Nikon DS Series Digital Cameras. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from Abroad The skyla HB1 Clinical Chemistry Analyzer provides clinical testing laboratories of medical facilities and clinics with an in-vitro clinical chemistry diagnostic tool that is compact, portable, and reports rapid results. The portable design of the clinical chemistry analyzer utilizes precision photometric measurement technology, combined with the single-use disposable reagent discs. The reagent disc contains a set of dry assay reagents that are used in the quantitative testing of various biomarkers in the blood sample. Delivery & Availability: Typically 14 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
| Out stock Little Sister Table Top Autoclave: This sterilizer is a table type in safety and auto controlled,which is designed for the unit of medical health, pharmacy, science and technology and mostly suitable for sterilizing of surgical,dental and eye instruments, glassware medicine, culture medium and biological dressing, food and other goods. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content | https://youtu.be/_ZV7XjEtCN0
From the Incubator to the Workstation – Stable Environment for the Best OutcomeEngineered for ultimate mobility and precision, the MIRI® M Chambers keep embryos in their ideal environment for extended periods, ensuring optimal conditions and more accurate results. Unlike conventional incubators that require frequent sample relocation, our innovative design preserves stability and integrity exactly where it's needed. This advanced approach minimizes environmental disruptions for embryos, which supports better IVF outcomesFeaturesProlonged Undisturbed Incubation
The MIRI® M Chambers create an ideal environment for embryos, ensuring they develop optimally. Unlike traditional incubators that need constant sample movement, this innovative design minimizes temperature and gas fluctuations, maintaining stable culture conditions. As a result, enhanced accuracy, improved outcomes, and greater success rates in IVF procedures can be expected.
Maximized Capacity for Efficiency and Scalability
The MIRI® M Docking Station improves workflow by accommodating up to 18 separate chambers, ensuring efficient organization and easy access. Its scalable design allows labs to manage multiple samples simultaneously while optimizing the vital space inside the laboratory.
Safety and Control in Sample Transportation
Thoughtfully crafted with an ergonomic surface, MIRI® M Chambers offer a firm grip for enhanced control and stability. This innovative design minimizes handling risks, ensures sample integrity, and empowers users with the confidence they need for successful handling procedures.
Enhanced Safety with Built-in Mix-up Prevention
The MIRI® M Chambers guarantee accurate patient identification and traceability through a clear graphical display that prominently presents patient information. This built-in safeguard reduces errors, streamlines workflow, and enhances sample security throughout the treatment process. By continuously assigning each chamber to the patient, users can freely change the chamber's position on the docking station without compromising traceability.
Advanced Alarm System
MIRI® M multiroom incubator provides information about alarm conditions through expansive pop-ups, icons, and colors on the Docking Station‘s and Chambers‘ screens, as well as backlights in the back panel.
Other Features
General SpecificationsMIRI® M Multiroom IVF Incubator Specifications
MIRI® M Chamber's Specifications
| Nikon SMZ745 Stereoscopic Microscope: Featuring a zoom magnification of 7.5X and a long working distance of 115mm, Nikon SMZ745 microscopes are well suited for both industrial and biomedical applications. The adoption of a new total reflection prism produces brighter images with higher contrast, and the anti-mold design allows the microscope to be used in environments where the temperature and humidity are high. The SMZ-745 trinocular model comes equipped with a microscope camera port and a built-in 0.55x c-mount adapter, permitting direct mounting of Nikon DS Series Digital Cameras.
Features:
| Skyla HB1 Clinical Chemistry Analyzer utilizes precision photometric measurement technology, combined with the single-use disposable reagent discs. The reagent disc contains a set of dry assay reagents that are used in the quantitative testing of various biomarkers in the blood sample. HB1 provides clinical testing laboratories of medical facilities and clinics with an in-vitro clinical chemistry diagnostic tool that is compact, portable, and reports rapid results. Only needs 200µl of blood (whole blood, serum or plasma is accepted.) and no sample preparation is required. All steps are designed automatically from checking the validity of reagent discs and cartridges to printing the results. Over 30 test parameters are allowed with fast analysis and results in 15 minutes. Internal Quality Control (IQC) with self-calibration of optical system, chemicals stability and interference alerts functions monitor and ensure the results with high accuracy.
Available Test Panels
Click Here To Download Catalogue | 800-1 electric centrifuge is widely used in hospitals, chemical and Biological Chemistry Laboratory for serum, plasma, immune qualitative analysis experiment. Has beautiful shape, large capacity, small volume, complete function. Its performance is stable, the speed is adjustable and can automatically adjust the balance, low temperature, high efficiency and wide applicability and advantages.
Technical Specifications:
Click Here To Download Catalogue | Little Sister Table Top Autoclave: This sterilizer is a table type in safety and auto controlled,which is designed for the unit of medical health, pharmacy, science and technology and mostly suitable for sterilizing of surgical,dental and eye instruments, glassware medicine, culture medium and biological dressing, food and other goods.
Features:
| This Automatic Pipette will help you get your lab up and running in no time—at a great price!
Features:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||














Reviews
There are no reviews yet.