DynaMesh-CICAT
$0.00
Shipped From Abroad
DynaMesh-CICAT and DynaMesh-CICAT visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the prevention of incisional hernias, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

Anti-Slip Surface
The devices have an anti-slip surface that ensures stable positioning of the mesh with reduced fixation compared to other devices available on the market. [132PB, 133PB, 134]

Dynamometric Positioning
For the correct dynamometric positioning of the device, ensure that the green or black marking strips are in a cranio-caudal orientation.

High Effective Porosity
The devices have a high effective porosity, which is known to reduce inflammation and the risk of excessive scar formation. [103P, TR33, TR35]

The device must not be placed partially or completely intraperitoneally.
Specifications
| Product | DynaMesh®-CICAT (1) DynaMesh®-CICAT visible (2) |
| Surgical Treatment | Epigastric Hernias / Umbilical Hernias / Incisional Hernias Prevention: Incisional Hernia |
| Surgical Approach | Minimally Invasive / Open |
| Mesh Position | Extraperitoneal (onlay, sublay and/or preperitoneal) |
| Fixation | Sutures / Tacks / Tissue Adhesives |
| Marking Strips | Green (1) / Black (2) |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) |
PVDF
|
| Biocompatibility |
|
| Ageing Resistance |
|
| Dynamometric Properties |
|
| Tear Propagation Resistance |
|
| Anti-Slip Surface |
|
| Effective Porosity | |
| Klinge’s Mesh Classification | Class 1a [102P, TR33, TR35] |
| DynaMesh®-CICAT implants must be placed extraperitoneally in onlay, sublay and/or preperitoneal mesh position. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
Quick Comparison
| DynaMesh-CICAT remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | Littmann Classic III Stethoscope remove | Spinal Epidural Pack remove | Oxygen Regulator (Japan Technology) remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-CICAT remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | Littmann Classic III Stethoscope remove | Spinal Epidural Pack remove | Oxygen Regulator (Japan Technology) remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | ||||||||||||||||||||||||||||||||||||||||||||
| Image |  | 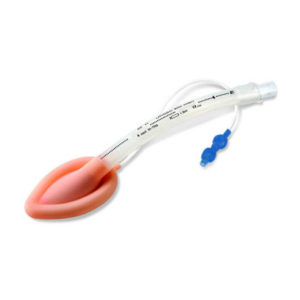 |  |  |  | 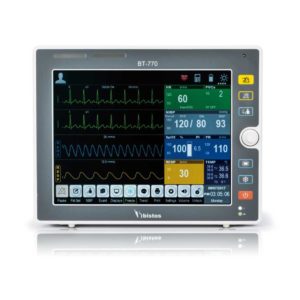 | ||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-5 | SF1033560084-20 | SF1033560084-34 | SF1033560084-25 | SF1033560084-48 | SF1033560059-1 | ||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $12.75 | $34.00 |
| $17.00 | $902.00 | ||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh-CICAT and DynaMesh-CICAT visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the prevention of incisional hernias, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In stock
| In Stock
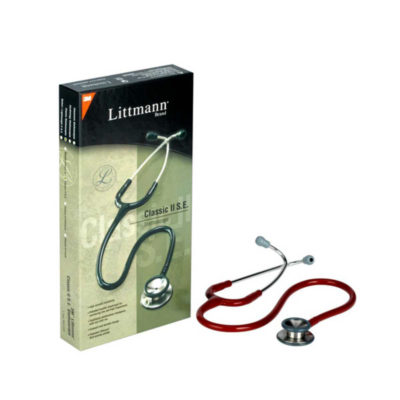
The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
It is for performing spinal/epidural or combined spinal/epidural or never-block loco-regional anaesthesia on patinet in clinical surgery.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
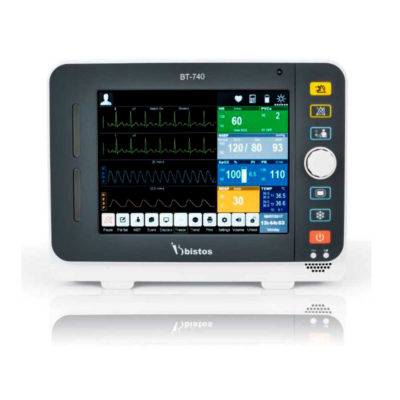
| Shipped from Abroad The Bistos BT-770 patient monitor is equipped with a 12.1" touchscreen display, which allows for an easy operation and readability with a powerful rechargeable battery guaranteeing a continuous operation of 5 hours to monitor ECG, SpO2, NIBP, temperature and respiration Delivery & Availability: Typically 14 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | ||||||||||||||||||||||||||||||||||||||||||||
| Content |
Specifications
|
| The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
Full adult and pediatric auscultation is available through its dual head chestpiece simply by rotating the central spline to alternate between the two sides. The more compact one piece diaphragm and rim assemblies have further increased acoustic sensitivity. The binaurals have been redesigned with larger diameter ear tubes and a thicker single lumen binaural to transfer sound more effectively. The selector spline is now recessed to allow a flush fit with the binaural tube and now features a visual indicator for easy identification of the operational diaphragm.
Features
| It is for performing spinal/epidural or combined spinal/epidural or never-block loco-regional anaesthesia on patinet in clinical surgery.
Component:
|
|
Bistos BT-770 is a 12.1" touchscreen patient monitor designed for easy operations.
SPECIFICATIONS
Click Here To Download Catalogue | ||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||
| Additional information |






Reviews
There are no reviews yet.