DynaMesh-CICAT
$0.00
Shipped From Abroad
DynaMesh-CICAT and DynaMesh-CICAT visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the prevention of incisional hernias, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

Anti-Slip Surface
The devices have an anti-slip surface that ensures stable positioning of the mesh with reduced fixation compared to other devices available on the market. [132PB, 133PB, 134]

Dynamometric Positioning
For the correct dynamometric positioning of the device, ensure that the green or black marking strips are in a cranio-caudal orientation.

High Effective Porosity
The devices have a high effective porosity, which is known to reduce inflammation and the risk of excessive scar formation. [103P, TR33, TR35]

The device must not be placed partially or completely intraperitoneally.
Specifications
| Product | DynaMesh®-CICAT (1) DynaMesh®-CICAT visible (2) |
| Surgical Treatment | Epigastric Hernias / Umbilical Hernias / Incisional Hernias Prevention: Incisional Hernia |
| Surgical Approach | Minimally Invasive / Open |
| Mesh Position | Extraperitoneal (onlay, sublay and/or preperitoneal) |
| Fixation | Sutures / Tacks / Tissue Adhesives |
| Marking Strips | Green (1) / Black (2) |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) |
PVDF
|
| Biocompatibility |
|
| Ageing Resistance |
|
| Dynamometric Properties |
|
| Tear Propagation Resistance |
|
| Anti-Slip Surface |
|
| Effective Porosity | |
| Klinge’s Mesh Classification | Class 1a [102P, TR33, TR35] |
| DynaMesh®-CICAT implants must be placed extraperitoneally in onlay, sublay and/or preperitoneal mesh position. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
Quick Comparison
| DynaMesh-CICAT remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | Electric Suction Machine remove | Little Sister Table Top Autoclave remove | Oxygen Concentrator 3Ltr. remove | Littman Stethoscope remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-CICAT remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | Electric Suction Machine remove | Little Sister Table Top Autoclave remove | Oxygen Concentrator 3Ltr. remove | Littman Stethoscope remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  | 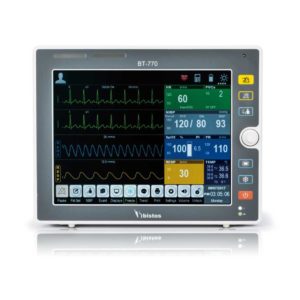 |  |  |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-5 | SF1033560059-1 | SF1033560084-16 | SF1033560084-46 | SF1033560084-40 | SF1033560084-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $902.00 | $152.00 | $1,350.00 | $273.00 | $13.20 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh-CICAT and DynaMesh-CICAT visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the prevention of incisional hernias, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| Shipped from Abroad The Bistos BT-770 patient monitor is equipped with a 12.1" touchscreen display, which allows for an easy operation and readability with a powerful rechargeable battery guaranteeing a continuous operation of 5 hours to monitor ECG, SpO2, NIBP, temperature and respiration Delivery & Availability: Typically 14 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
Power consumption: 120W
Maximum negative pressure: ≥0.09 MPa
Pump rate: ≥20 L/min
Noise: ≤60 dB
Continuous Working Time: 0-30 min
Bottle volume: 2500 ml, 2 pcs
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Out stock Little Sister Table Top Autoclave: This sterilizer is a table type in safety and auto controlled,which is designed for the unit of medical health, pharmacy, science and technology and mostly suitable for sterilizing of surgical,dental and eye instruments, glassware medicine, culture medium and biological dressing, food and other goods. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
| In Stock
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Specifications
|
Bistos BT-770 is a 12.1" touchscreen patient monitor designed for easy operations.
SPECIFICATIONS
Click Here To Download Catalogue | Specification :
Voltage: AC 220V, 50Hz or 110V, 60Hz
Power consumption: 120 W
Maximum negative pressure: ≥0.09 MPa
Negative pressure range: 0.02 MPa to Max negative pressure
Pump rate: ≥20 L/min
Noise: ≤60 dB
Continuous Working Time: 0-30 min
Rest Time≥30min
Bottle volume: 2500 ml, 2 pcs
Accessory: pedal switch (1pc), power switch(1pc),
filter(2 pcs), suction pipe(1pc, 2m in length, silica gel),
celiac suction pipe(1pc, 31cm in length, chrome copper)
Package: 1pc/carton
Box Size : 46.5*41.5*64.5cm
Net Weight: 11.5KGS
Gross Weight:13.8 KGS
Click Here To Download Catalogue | Little Sister Table Top Autoclave: This sterilizer is a table type in safety and auto controlled,which is designed for the unit of medical health, pharmacy, science and technology and mostly suitable for sterilizing of surgical,dental and eye instruments, glassware medicine, culture medium and biological dressing, food and other goods.
Features:
| Our anesthesia system is a precision crafted, life-sustaining device. When moving to an O2 concentrator from tanks, you should expect the same attention to detail.
A full 8lpm O2 flowrate capability - gives better performance at lower flow rates
20psi pressure output - your anesthesia system was designed to work from pressure
O2 concentrations average 93% +/- 3%
Built-in Low O2 Concentration Alarm
Small footprint of 15"x15"x22"
Remarkably quiet
Save money over time vs. tanks
Eliminate contamination hazards of tanks coming and going in your facility
Works with ALL manufacturer's brands of anesthesia systems
| Littman Stethoscope Features:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||









Reviews
There are no reviews yet.