DynaMesh-ENDOLAP 3D
$0.00
Shipped From Abroad
DynaMesh-ENDOLAP 3D and DynaMesh-ENDOLAP 3D visible implants are intended for the surgical treatment of inguinal or femoral hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

3D Shape
The device is a preformed three-dimensional mesh with a green central longitudinal marking, a green edge marking and centrally attached green filament ends.

Standardised Positioning
The device must be placed in such a way that the central green longitudinal marking is aligned with the inguinal ligament, the green central filament ends are located in projection to the junction of the inferior epigastric blood vessels to the external iliac blood vessels, and the green edge marking points dorsocaudally. The green filament ends must point to the user.
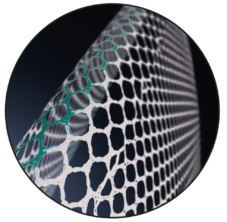
Varying Pore Size
The pore size of the device varies laterally to the longitudinal marking.
Product Range
When selecting and cutting the device, sufficient defect overlap must be ensured to minimise the likelihood of the hernia recurring.
| DynaMesh®-ENDOLAP 3D | 09 cm x 14 cm | PV130914F1/F3 |
| DynaMesh®-ENDOLAP 3D | 10 cm x 15 cm regular | PV131015F1/F3 |
| DynaMesh®-ENDOLAP 3D | 12 cm x 17 cm | PV131217F1/F5 |
| DynaMesh®-ENDOLAP 3D visible | 09 cm x 14 cm | PV120914F1/F3 |
| DynaMesh®-ENDOLAP 3D visible | 10 cm x 15 cm | PV121015F1/F3 |
| DynaMesh®-ENDOLAP 3D visible | 12 cm x 17 cm | PV121217F1 |
| FX = X unit(s)/box (e.g. F3 = 3 unit(s)/box) |
Can be used both for the right and the left side.
Features
| Product | DynaMesh®-ENDOLAP 3D (1) DynaMesh®-ENDOLAP 3D visible (2) |
| Surgical Treatment | Inguinal Hernias / Femoral Hernias |
| Surgical Approach | Laparoscopic / Endoscopic |
| Surgical Technique | TAPP / TEP |
| Mesh Position | Preperitoneal (Posterior) |
| Fixation | (None)* / Sutures / Tissue Adhesives / Tacks |
| Green Central and Edge Marking | |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) (2) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) |
PVDF
|
| Biocompatibility | |
| Ageing Resistance |
|
| Tear Propagation Resistance | |
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR23] |
| * Based on current knowledge, with the exception of large direct inguinal hernias (European Hernia Society classification: M3), it appears possible to dispense with any form of fixation. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#”(see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
Quick Comparison
| DynaMesh-ENDOLAP 3D remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | Littman Stethoscope remove | Elastomeric Pump (Disposable Infusion Pump) with PCA remove | Bettermed BT667E Electric Operating Table remove | 18Ltrs Bucket Autoclave remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-ENDOLAP 3D remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | Littman Stethoscope remove | Elastomeric Pump (Disposable Infusion Pump) with PCA remove | Bettermed BT667E Electric Operating Table remove | 18Ltrs Bucket Autoclave remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image | 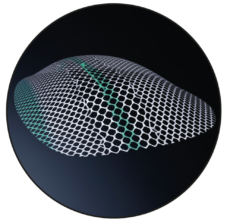 |  |  |  |  |  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560084-37 | SF1033560084-5 | SF1033560084-18 | SF1033560084-50 | SF1033560084-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $45.00 | $13.20 |
| $2,178.00 |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh-ENDOLAP 3D and DynaMesh-ENDOLAP 3D visible implants are intended for the surgical treatment of inguinal or femoral hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In stock
Omron M2 Upper Arm Blood Pressure Monitor Medium CufF: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage. zWith this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| In Stock
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
This product make the tension of silicon capsule as the driving force to control the flow by micro porous tube, it was pre-selected by doctor base on patients'situation, and then and then handed over to the patients ' self- management “to relieve or ease the pain
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
This operation table is used for the surgeries of chest ,abdomen ,ophthalmology,obstetrics,and urology.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Varying Pore Size The pore size of the device varies laterally to the longitudinal marking.
Features
| Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage.With this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| Littman Stethoscope Features:
| This product make the tension of silicon capsule as the driving force to control the flow by micro porous tube, it was pre-selected by doctor base on patients'situation, and then and then handed over to the patients ' self- management “to relieve or ease the pain Product Components: one-way valve medicated device, elastic liquid storage device, liquid medicine filter, current limiting device, automatic liquid feeding device ( CBI & PCA ), pipe and the external connector; Product Features: 1. The continued to liquid (CBI) make the drug-stored sac mechanical stretch as a driving force, limited the flow through the micro porous tube to the patient safely, effectively, slightly, continuous uniform injection of liquid to analgesic effect; extrusion pump body does not affect the liquid normal velocity; liquid filtering device which can control the particles enter the body better; 2. Continued to liquid + self control to liquid ( CBI+PCA ) make the drug-stored sac mechanical stretch as a driving force, limited the flow through the micro porous tube to the patient safely, effectively, slightly, continuous uniform injection of liquid to analgesic effect; Automatic medicated function is the patients can medicate according to pain independently. Extrusion pump body does not affect the liquid normal velocity; liquid filtering device which can control the particles enter the body better; How to use: 1. Get analgesia pump out from sterile packaging, removing the cap of medicine inlet and outlet; pour the analgesic liquid in the liquid storage device, draw out the air from the liquid storage. 2. Finish medicating, waiting for the liquid outflow from the medicine outlet; please clamp tightly to stop flowing for preparation. 3. Control keys (PCA key) is a function key to add the medicine base on continue to medicated by patients' self-controlled. So the patients can self-control the PCA key under the guidance of doctors, according to the needs while they are in treatment. At the same time, doctor should fill in PCA treatment card, so that they can analyze the condition from PCA additional times. | This operation table is used for the surgeries of chest, abdomen, ophthalmology, obstetricsand urology, etc.It has the following advantages:
●The board can shift longitudinally. It is made of Transparent material material.it can be X-rayed or scan when using along with C-ARM .
●The board is controlled by electric handspike to move up and down, lean from left to right and move back and forth.
●The leg board is dismantable. It is manual rotation and could be ooutreached with the force from the spring and gravity, convenient for urology surgery.
Technical Specification
| Features:
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information |







Reviews
There are no reviews yet.