DynaMesh-ENDOLAP 3D
$0.00
Shipped From Abroad
DynaMesh-ENDOLAP 3D and DynaMesh-ENDOLAP 3D visible implants are intended for the surgical treatment of inguinal or femoral hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

3D Shape
The device is a preformed three-dimensional mesh with a green central longitudinal marking, a green edge marking and centrally attached green filament ends.

Standardised Positioning
The device must be placed in such a way that the central green longitudinal marking is aligned with the inguinal ligament, the green central filament ends are located in projection to the junction of the inferior epigastric blood vessels to the external iliac blood vessels, and the green edge marking points dorsocaudally. The green filament ends must point to the user.
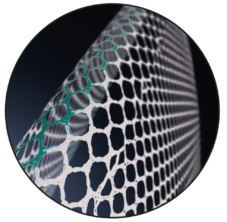
Varying Pore Size
The pore size of the device varies laterally to the longitudinal marking.
Product Range
When selecting and cutting the device, sufficient defect overlap must be ensured to minimise the likelihood of the hernia recurring.
| DynaMesh®-ENDOLAP 3D | 09 cm x 14 cm | PV130914F1/F3 |
| DynaMesh®-ENDOLAP 3D | 10 cm x 15 cm regular | PV131015F1/F3 |
| DynaMesh®-ENDOLAP 3D | 12 cm x 17 cm | PV131217F1/F5 |
| DynaMesh®-ENDOLAP 3D visible | 09 cm x 14 cm | PV120914F1/F3 |
| DynaMesh®-ENDOLAP 3D visible | 10 cm x 15 cm | PV121015F1/F3 |
| DynaMesh®-ENDOLAP 3D visible | 12 cm x 17 cm | PV121217F1 |
| FX = X unit(s)/box (e.g. F3 = 3 unit(s)/box) |
Can be used both for the right and the left side.
Features
| Product | DynaMesh®-ENDOLAP 3D (1) DynaMesh®-ENDOLAP 3D visible (2) |
| Surgical Treatment | Inguinal Hernias / Femoral Hernias |
| Surgical Approach | Laparoscopic / Endoscopic |
| Surgical Technique | TAPP / TEP |
| Mesh Position | Preperitoneal (Posterior) |
| Fixation | (None)* / Sutures / Tissue Adhesives / Tacks |
| Green Central and Edge Marking | |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) (2) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) |
PVDF
|
| Biocompatibility | |
| Ageing Resistance |
|
| Tear Propagation Resistance | |
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR23] |
| * Based on current knowledge, with the exception of large direct inguinal hernias (European Hernia Society classification: M3), it appears possible to dispense with any form of fixation. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#”(see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
Quick Comparison
| DynaMesh-ENDOLAP 3D remove | Ambu Bag with Reservior remove | Oxygen Regulator (Japan Technology) remove | Portable Phelgm Suction Unit remove | Bettermed BT666EPZ Multi-function Electric Delivery Bed remove | Bistos BT- 720 Patient Monitor remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-ENDOLAP 3D remove | Ambu Bag with Reservior remove | Oxygen Regulator (Japan Technology) remove | Portable Phelgm Suction Unit remove | Bettermed BT666EPZ Multi-function Electric Delivery Bed remove | Bistos BT- 720 Patient Monitor remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image | 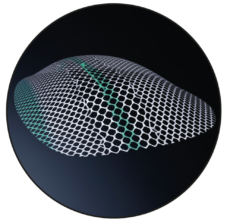 | 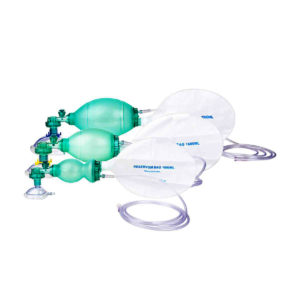 |  | 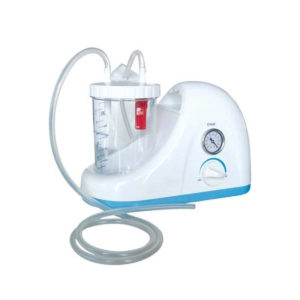 | 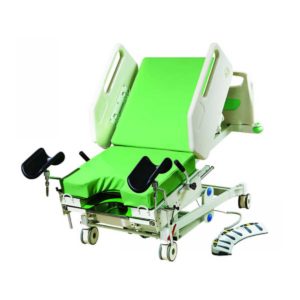 |  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560084-41 | SF1033560084-48 | SF1033560084-10 | SF1033560084-51 | SF1033560059-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $17.00 | $17.00 | $54.50 | $2,024.00 | $330.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh-ENDOLAP 3D and DynaMesh-ENDOLAP 3D visible implants are intended for the surgical treatment of inguinal or femoral hernias and permanently bridge and reinforce the soft tissue in the groin region in the area of the hernia defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In stock
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
| In stock
| In Stock Portable Phelgm Suction Unit: Portable phlegm suction is designed based on developing orientation of similar products at home and abroad. It is a new generation of oil free lubrication suction device, suitable for use by the patient who has difficult in phlegm removal due to illness, coma and operations, as well as for aspiration liquid as pus and blood during clinical practice. It is commonly applied for using in the emergency room, operation room and for nursing and home health care. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
Function:Backrest tilting From 0°to 75° ±5°
Hi-lo function
Reverse:14°±1°
Trendelenburg:12°±1°
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from abroad Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer. Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Varying Pore Size The pore size of the device varies laterally to the longitudinal marking.
Features
|
Ambu Bag with Reservior: The ventilation bag is highly reactive with fast recoil and excellent stroke volume which help relieving fatigue to the rescuer. The thin-walled compression bag provides an excellent feel of lung compliance and helps avoid excessive ventilation that could damage the lungs.
Features:
|
| Portable Phelgm Suction Unit: Portable phlegm suction is designed based on developing orientation of similar products at home and abroad. It is a new generation of oil free lubrication suction device, suitable for use by the patient who has difficult in phlegm removal due to illness, coma and operations, as well as for aspiration liquid as pus and blood during clinical practice. It is commonly applied for using in the emergency room, operation room and for nursing and home health care.
Features:
|
| Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer.
Features:
Click Here To Download Catalogue | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||









Reviews
There are no reviews yet.