DynaMesh-IPOM
$0.00
Shipped From Abroad
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the treatment of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are primarily made of polyvinylidene fluoride (PVDF).
The mesh implants are warp-knitted using coloured and uncoloured polyvinylidene fluoride (PVDF) monofilaments and uncoloured polypropylene (PP) monofilaments.
Positioning
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by the green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
The mesh implant must be placed in such a way that the green filament ends are always oriented towards the abdominal wall. At the same time, the mesh implant must be oriented so that the green filament ends are cranial or caudal.
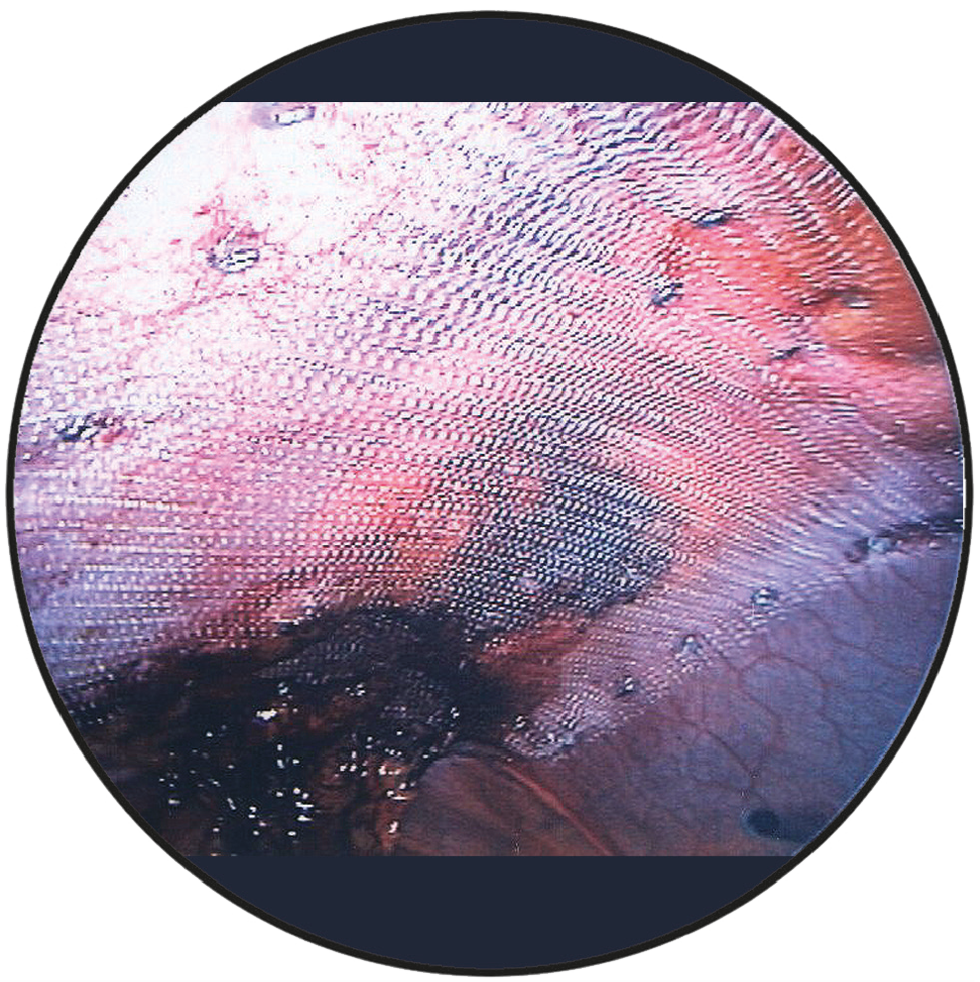
Intraoperative view

Specifications
| Product | DynaMesh®-IPOM (1) DynaMesh®-IPOM visible (2) |
| Surgical Treatment | Umbilical Hernias / Epigastric Hernias / Incisional Hernias / Parastomal Hernias |
| Surgical Approach | Minimally Invasive / Open |
| Mesh Position | Intraperitoneal* according to the intraperitoneal onlay mesh technique (IPOM). |
| Fixation | Suture / Tacks / No Fibrin Glue |
| Green Filament Ends | |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 85% (w/w) (1) (2) – Polypropylene (PP) (CAS 9003-07-0) < 13% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) (2) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymers (Monofilament) | PVDF, PP |
| Biocompatibility |
|
| Ageing Resistance |
|
| Tear Propagation Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR64] |
| * In particular cases with an extraperitoneal mesh position in which there is a risk of contact between the mesh implant and the intestine, the device may also be placed extraperitoneally in onlay, sublay and/or preperitoneal mesh position. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
Quick Comparison
| DynaMesh-IPOM remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | Ambu Oval Silicone resuscitator (Ambu Bag) remove | 18Ltrs Bucket Autoclave remove | Littmann Classic III Stethoscope remove | Oxygen Concentrator remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-IPOM remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | Ambu Oval Silicone resuscitator (Ambu Bag) remove | 18Ltrs Bucket Autoclave remove | Littmann Classic III Stethoscope remove | Oxygen Concentrator remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  |  |  |  |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-4 | SF1033560084-37 | SF1033560084-62 | SF1033560084-7 | SF1033560084-34 | SF1033560084-31 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $45.00 | $27.00 |
| $34.00 | $350.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | In stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | In stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the treatment of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In stock
Omron M2 Upper Arm Blood Pressure Monitor Medium CufF: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage. zWith this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| In Stock The Ambu Oval Silicone resuscitator is designed for manual ventilation of neonates though to adults. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
| In Stock
The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are primarily made of polyvinylidene fluoride (PVDF).
The mesh implants are warp-knitted using coloured and uncoloured polyvinylidene fluoride (PVDF) monofilaments and uncoloured polypropylene (PP) monofilaments.
Positioning
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by the green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
The mesh implant must be placed in such a way that the green filament ends are always oriented towards the abdominal wall. At the same time, the mesh implant must be oriented so that the green filament ends are cranial or caudal.
Specifications
| Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage.With this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| The Ambu Oval Silicone resuscitator is designed for manual ventilation of neonates though to adults.
Features
* International guidelines recommend that all manual resuscitators for infant and pediatric patients should be equipped with a pressure limitation mechanism so the airway pressure does not exceed 45 cm H2O. | Features:
| The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
Full adult and pediatric auscultation is available through its dual head chestpiece simply by rotating the central spline to alternate between the two sides. The more compact one piece diaphragm and rim assemblies have further increased acoustic sensitivity. The binaurals have been redesigned with larger diameter ear tubes and a thicker single lumen binaural to transfer sound more effectively. The selector spline is now recessed to allow a flush fit with the binaural tube and now features a visual indicator for easy identification of the operational diaphragm.
Features
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information |









Reviews
There are no reviews yet.