DynaMesh-IPOM
$0.00
Shipped From Abroad
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the treatment of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are primarily made of polyvinylidene fluoride (PVDF).
The mesh implants are warp-knitted using coloured and uncoloured polyvinylidene fluoride (PVDF) monofilaments and uncoloured polypropylene (PP) monofilaments.
Positioning
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by the green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
The mesh implant must be placed in such a way that the green filament ends are always oriented towards the abdominal wall. At the same time, the mesh implant must be oriented so that the green filament ends are cranial or caudal.
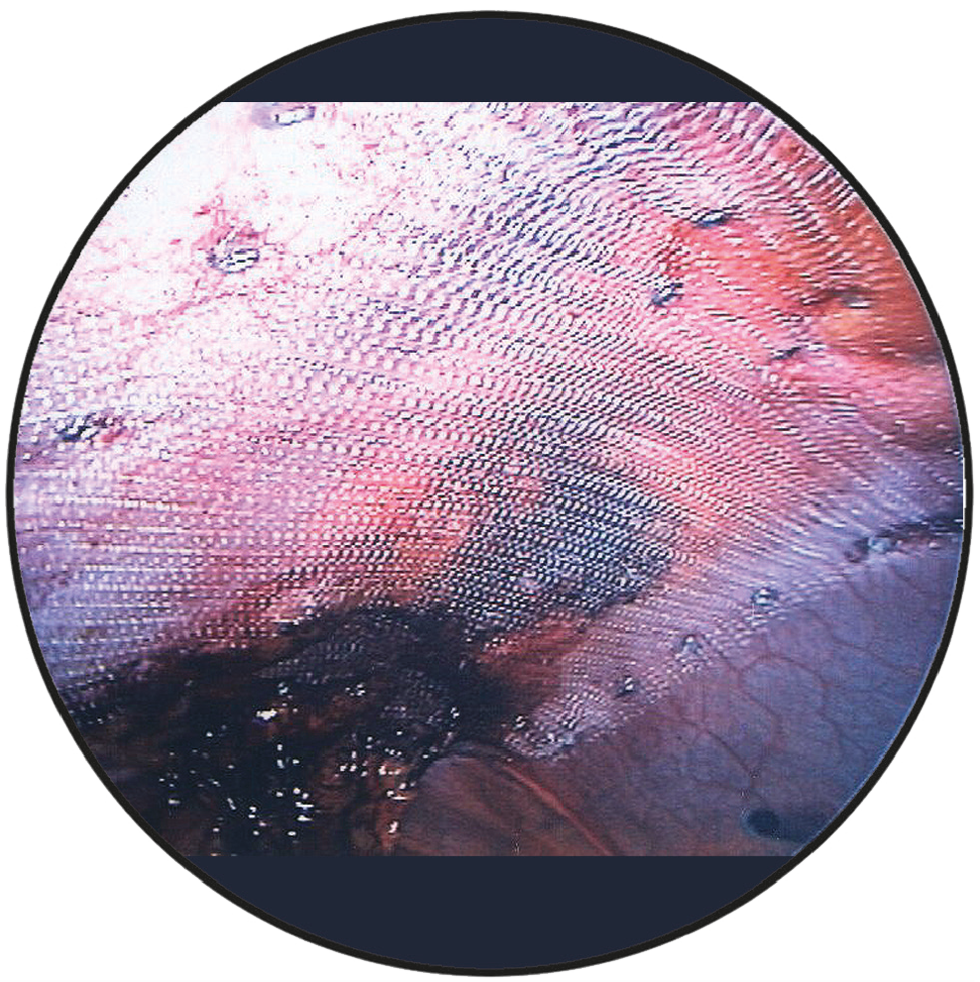
Intraoperative view

Specifications
| Product | DynaMesh®-IPOM (1) DynaMesh®-IPOM visible (2) |
| Surgical Treatment | Umbilical Hernias / Epigastric Hernias / Incisional Hernias / Parastomal Hernias |
| Surgical Approach | Minimally Invasive / Open |
| Mesh Position | Intraperitoneal* according to the intraperitoneal onlay mesh technique (IPOM). |
| Fixation | Suture / Tacks / No Fibrin Glue |
| Green Filament Ends | |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 85% (w/w) (1) (2) – Polypropylene (PP) (CAS 9003-07-0) < 13% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) (2) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymers (Monofilament) | PVDF, PP |
| Biocompatibility |
|
| Ageing Resistance |
|
| Tear Propagation Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR64] |
| * In particular cases with an extraperitoneal mesh position in which there is a risk of contact between the mesh implant and the intestine, the device may also be placed extraperitoneally in onlay, sublay and/or preperitoneal mesh position. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
Quick Comparison
| Settings | DynaMesh-IPOM remove | Spinal Epidural Pack remove | Bistos BT- 720 Patient Monitor remove | Little Sister Table Top Autoclave remove | Oxygen Concentrator 3Ltr. remove | Bettermed BT666EPZ Multi-function Electric Delivery Bed remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-IPOM remove | Spinal Epidural Pack remove | Bistos BT- 720 Patient Monitor remove | Little Sister Table Top Autoclave remove | Oxygen Concentrator 3Ltr. remove | Bettermed BT666EPZ Multi-function Electric Delivery Bed remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  |  |  |  |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-4 | SF1033560084-25 | SF1033560059-8 | SF1033560084-46 | SF1033560084-40 | SF1033560084-51 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
|
| $330.00 | $1,350.00 | $273.00 | $2,024.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the treatment of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In Stock
It is for performing spinal/epidural or combined spinal/epidural or never-block loco-regional anaesthesia on patinet in clinical surgery.
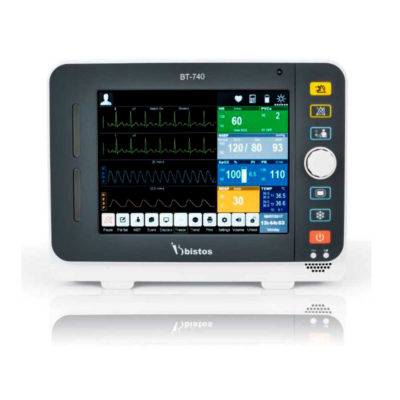
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from abroad Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer. Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Out stock Little Sister Table Top Autoclave: This sterilizer is a table type in safety and auto controlled,which is designed for the unit of medical health, pharmacy, science and technology and mostly suitable for sterilizing of surgical,dental and eye instruments, glassware medicine, culture medium and biological dressing, food and other goods. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
| In Stock
Function:Backrest tilting From 0°to 75° ±5°
Hi-lo function
Reverse:14°±1°
Trendelenburg:12°±1°
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are primarily made of polyvinylidene fluoride (PVDF).
The mesh implants are warp-knitted using coloured and uncoloured polyvinylidene fluoride (PVDF) monofilaments and uncoloured polypropylene (PP) monofilaments.
Positioning
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by the green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
The mesh implant must be placed in such a way that the green filament ends are always oriented towards the abdominal wall. At the same time, the mesh implant must be oriented so that the green filament ends are cranial or caudal.
Specifications
| It is for performing spinal/epidural or combined spinal/epidural or never-block loco-regional anaesthesia on patinet in clinical surgery.
Component:
| Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer.
Features:
Click Here To Download Catalogue | Little Sister Table Top Autoclave: This sterilizer is a table type in safety and auto controlled,which is designed for the unit of medical health, pharmacy, science and technology and mostly suitable for sterilizing of surgical,dental and eye instruments, glassware medicine, culture medium and biological dressing, food and other goods.
Features:
| Our anesthesia system is a precision crafted, life-sustaining device. When moving to an O2 concentrator from tanks, you should expect the same attention to detail.
A full 8lpm O2 flowrate capability - gives better performance at lower flow rates
20psi pressure output - your anesthesia system was designed to work from pressure
O2 concentrations average 93% +/- 3%
Built-in Low O2 Concentration Alarm
Small footprint of 15"x15"x22"
Remarkably quiet
Save money over time vs. tanks
Eliminate contamination hazards of tanks coming and going in your facility
Works with ALL manufacturer's brands of anesthesia systems
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||





Reviews
There are no reviews yet.