DynaMesh-IPOM
$0.00
Shipped From Abroad
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the treatment of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are primarily made of polyvinylidene fluoride (PVDF).
The mesh implants are warp-knitted using coloured and uncoloured polyvinylidene fluoride (PVDF) monofilaments and uncoloured polypropylene (PP) monofilaments.
Positioning
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by the green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
The mesh implant must be placed in such a way that the green filament ends are always oriented towards the abdominal wall. At the same time, the mesh implant must be oriented so that the green filament ends are cranial or caudal.
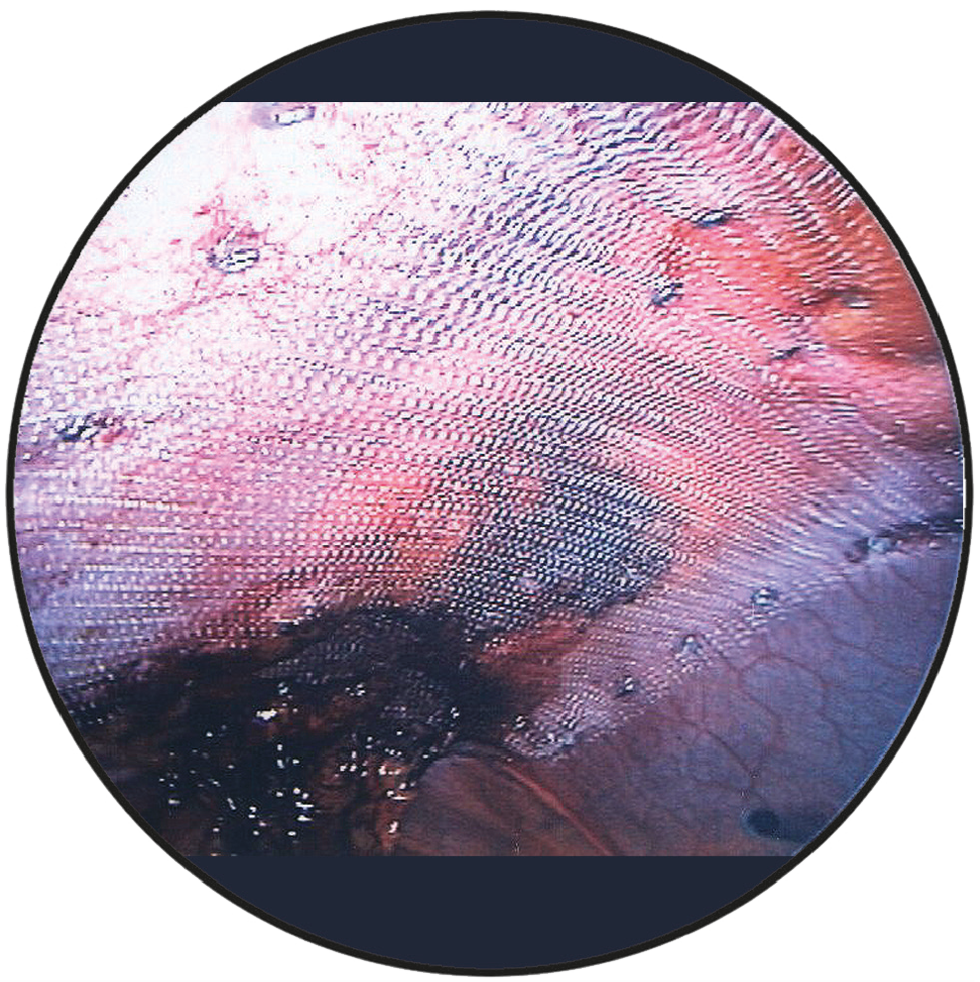
Intraoperative view

Specifications
| Product | DynaMesh®-IPOM (1) DynaMesh®-IPOM visible (2) |
| Surgical Treatment | Umbilical Hernias / Epigastric Hernias / Incisional Hernias / Parastomal Hernias |
| Surgical Approach | Minimally Invasive / Open |
| Mesh Position | Intraperitoneal* according to the intraperitoneal onlay mesh technique (IPOM). |
| Fixation | Suture / Tacks / No Fibrin Glue |
| Green Filament Ends | |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 85% (w/w) (1) (2) – Polypropylene (PP) (CAS 9003-07-0) < 13% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) (2) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymers (Monofilament) | PVDF, PP |
| Biocompatibility |
|
| Ageing Resistance |
|
| Tear Propagation Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR64] |
| * In particular cases with an extraperitoneal mesh position in which there is a risk of contact between the mesh implant and the intestine, the device may also be placed extraperitoneally in onlay, sublay and/or preperitoneal mesh position. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
Quick Comparison
| DynaMesh-IPOM remove | Littmann Classic III Stethoscope remove | Bettermed BT667E Electric Operating Table remove | Oxygen Concentrator 3Ltr. remove | De Lineb Oxygen Face Mask remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-IPOM remove | Littmann Classic III Stethoscope remove | Bettermed BT667E Electric Operating Table remove | Oxygen Concentrator 3Ltr. remove | De Lineb Oxygen Face Mask remove | Intersurgical Solus i-gel LMA (Size; 2, 3, 4 & 5) remove | ||||||||||||||||||||||||||||||||||||||||
| Image |  |  |  |  |  |  | ||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-4 | SF1033560084-34 | SF1033560084-50 | SF1033560084-40 | SF1033560084-39 | SF1033560084-20 | ||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $34.00 | $2,178.00 | $273.00 | $1.40 | $12.75 | ||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the treatment of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In Stock
The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
This operation table is used for the surgeries of chest ,abdomen ,ophthalmology,obstetrics,and urology.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
| In stock
De Lineb Oxygen Face Mask: This mask provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask).
| In stock
| ||||||||||||||||||||||||||||||||||||||||
| Content |
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are primarily made of polyvinylidene fluoride (PVDF).
The mesh implants are warp-knitted using coloured and uncoloured polyvinylidene fluoride (PVDF) monofilaments and uncoloured polypropylene (PP) monofilaments.
Positioning
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by the green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
The mesh implant must be placed in such a way that the green filament ends are always oriented towards the abdominal wall. At the same time, the mesh implant must be oriented so that the green filament ends are cranial or caudal.
Specifications
| The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
Full adult and pediatric auscultation is available through its dual head chestpiece simply by rotating the central spline to alternate between the two sides. The more compact one piece diaphragm and rim assemblies have further increased acoustic sensitivity. The binaurals have been redesigned with larger diameter ear tubes and a thicker single lumen binaural to transfer sound more effectively. The selector spline is now recessed to allow a flush fit with the binaural tube and now features a visual indicator for easy identification of the operational diaphragm.
Features
| This operation table is used for the surgeries of chest, abdomen, ophthalmology, obstetricsand urology, etc.It has the following advantages:
●The board can shift longitudinally. It is made of Transparent material material.it can be X-rayed or scan when using along with C-ARM .
●The board is controlled by electric handspike to move up and down, lean from left to right and move back and forth.
●The leg board is dismantable. It is manual rotation and could be ooutreached with the force from the spring and gravity, convenient for urology surgery.
Technical Specification
| Our anesthesia system is a precision crafted, life-sustaining device. When moving to an O2 concentrator from tanks, you should expect the same attention to detail.
A full 8lpm O2 flowrate capability - gives better performance at lower flow rates
20psi pressure output - your anesthesia system was designed to work from pressure
O2 concentrations average 93% +/- 3%
Built-in Low O2 Concentration Alarm
Small footprint of 15"x15"x22"
Remarkably quiet
Save money over time vs. tanks
Eliminate contamination hazards of tanks coming and going in your facility
Works with ALL manufacturer's brands of anesthesia systems
| De Lineb Oxygen Face Mask: This mask provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask).
Features:
|
| ||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||
| Additional information |








Reviews
There are no reviews yet.