DynaMesh-IPOM
$0.00
Shipped From Abroad
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the treatment of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are primarily made of polyvinylidene fluoride (PVDF).
The mesh implants are warp-knitted using coloured and uncoloured polyvinylidene fluoride (PVDF) monofilaments and uncoloured polypropylene (PP) monofilaments.
Positioning
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by the green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
The mesh implant must be placed in such a way that the green filament ends are always oriented towards the abdominal wall. At the same time, the mesh implant must be oriented so that the green filament ends are cranial or caudal.
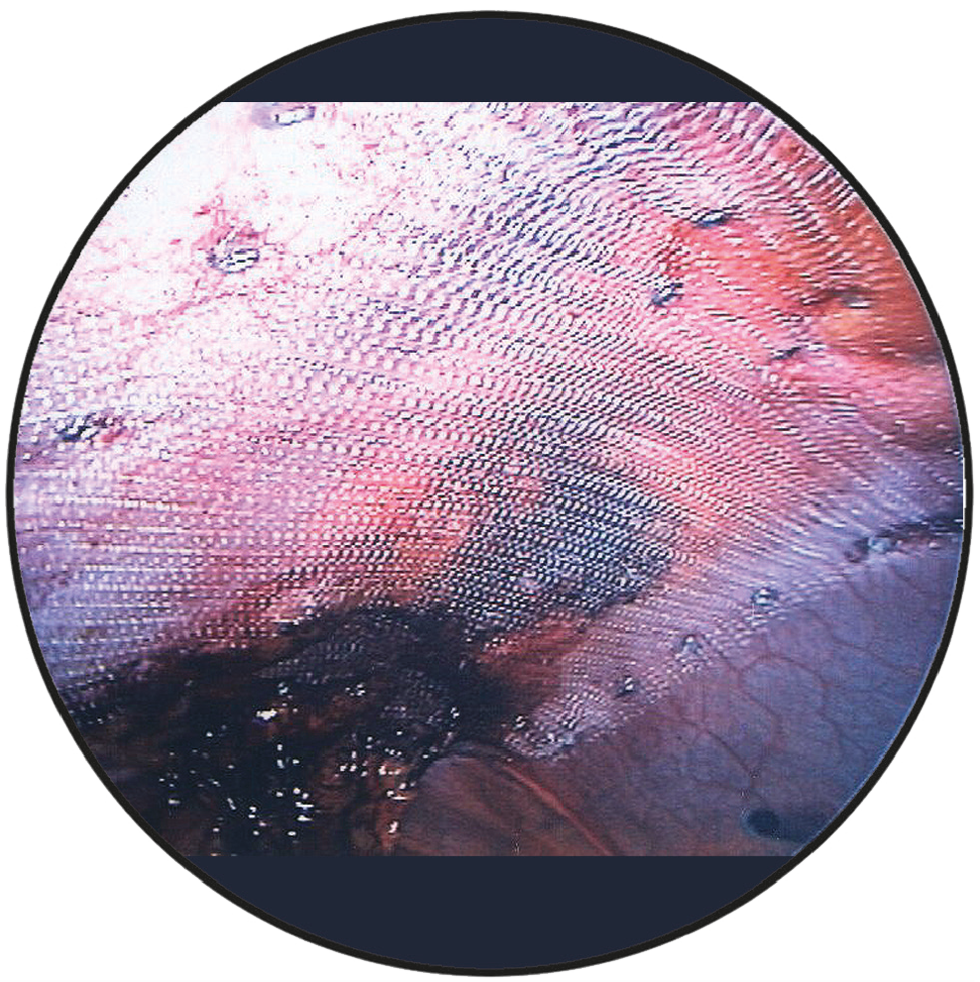
Intraoperative view

Specifications
| Product | DynaMesh®-IPOM (1) DynaMesh®-IPOM visible (2) |
| Surgical Treatment | Umbilical Hernias / Epigastric Hernias / Incisional Hernias / Parastomal Hernias |
| Surgical Approach | Minimally Invasive / Open |
| Mesh Position | Intraperitoneal* according to the intraperitoneal onlay mesh technique (IPOM). |
| Fixation | Suture / Tacks / No Fibrin Glue |
| Green Filament Ends | |
| Visible Technology | |
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 85% (w/w) (1) (2) – Polypropylene (PP) (CAS 9003-07-0) < 13% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) (2) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymers (Monofilament) | PVDF, PP |
| Biocompatibility |
|
| Ageing Resistance |
|
| Tear Propagation Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR64] |
| * In particular cases with an extraperitoneal mesh position in which there is a risk of contact between the mesh implant and the intestine, the device may also be placed extraperitoneally in onlay, sublay and/or preperitoneal mesh position. |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
Quick Comparison
| DynaMesh-IPOM remove | Electric Suction Machine remove | Bistos BT- 410 Medical Head Lamp remove | IBIS Neeo R9 Digital Surgical C-Arm remove | Little Sister Table Top Autoclave remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-IPOM remove | Electric Suction Machine remove | Bistos BT- 410 Medical Head Lamp remove | IBIS Neeo R9 Digital Surgical C-Arm remove | Little Sister Table Top Autoclave remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  |  |  |  |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-4 | SF1033560084-16 | SF1033560059-6 | SF1033560011-1 | SF1033560084-46 | SF1033560084-37 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $152.00 | $132.00 |
| $1,350.00 | $45.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are intended for the surgical treatment of epigastric hernias, umbilical or incisional hernias, and the treatment of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the abdominal wall defect.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In Stock
Power consumption: 120W
Maximum negative pressure: ≥0.09 MPa
Pump rate: ≥20 L/min
Noise: ≤60 dB
Continuous Working Time: 0-30 min
Bottle volume: 2500 ml, 2 pcs
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from abroad Bistos BT- 410 Medical Head Lamp - Head-worn light BT-410 provides not only comfortable wear but also convenient for use during examinations or operations. - Ultra bright LED light with High density LED - Easy to adjust head strap & an angle - Extensive LED lifetime (more than 50,000 hours) - More than 4 hours of continuous use - An additional astral LED lamp (optional) - Attachable loupe (optional) Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from Abroad Our Neeo “C” arms are easy to place, use and are specifically designed to be used in orthopedics, traumatology, abdominal surgery, urology, cardiology and operating rooms. Delivery & Availability: Typically 21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Out stock Little Sister Table Top Autoclave: This sterilizer is a table type in safety and auto controlled,which is designed for the unit of medical health, pharmacy, science and technology and mostly suitable for sterilizing of surgical,dental and eye instruments, glassware medicine, culture medium and biological dressing, food and other goods. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
Omron M2 Upper Arm Blood Pressure Monitor Medium CufF: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage. zWith this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants are primarily made of polyvinylidene fluoride (PVDF).
The mesh implants are warp-knitted using coloured and uncoloured polyvinylidene fluoride (PVDF) monofilaments and uncoloured polypropylene (PP) monofilaments.
Positioning
DynaMesh®-IPOM and DynaMesh®-IPOM visible implants have a parietal side and a visceral side. The parietal side is identified by the green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
The mesh implant must be placed in such a way that the green filament ends are always oriented towards the abdominal wall. At the same time, the mesh implant must be oriented so that the green filament ends are cranial or caudal.
Specifications
| Specification :
Voltage: AC 220V, 50Hz or 110V, 60Hz
Power consumption: 120 W
Maximum negative pressure: ≥0.09 MPa
Negative pressure range: 0.02 MPa to Max negative pressure
Pump rate: ≥20 L/min
Noise: ≤60 dB
Continuous Working Time: 0-30 min
Rest Time≥30min
Bottle volume: 2500 ml, 2 pcs
Accessory: pedal switch (1pc), power switch(1pc),
filter(2 pcs), suction pipe(1pc, 2m in length, silica gel),
celiac suction pipe(1pc, 31cm in length, chrome copper)
Package: 1pc/carton
Box Size : 46.5*41.5*64.5cm
Net Weight: 11.5KGS
Gross Weight:13.8 KGS
Click Here To Download Catalogue | Bistos BT- 410 Medical Head Lamp - Head-worn light BT-410 provides not only comfortable wear but also convenient for use during examinations or operations. - Ultra bright LED light with High density LED - Easy to adjust head strap & an angle - Extensive LED lifetime (more than 50,000 hours) - More than 4 hours of continuous use - An additional astral LED lamp (optional) - Attachable loupe (optional).
Features:
Illumination Uniformity:
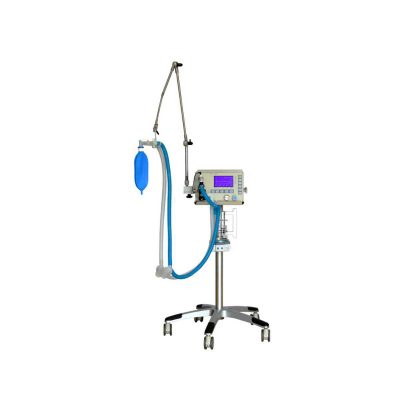
Click Here To Download Catalogue | Our Neeo “C” arms are easy to place, use and are specifically designed to be used in orthopedics, traumatology, abdominal surgery, urology, cardiology and operating rooms.
Using Neeo with the RTP (Real Time Processing) option it is possible to perform vascular, urological and cardiological diagnostics. One of the main functions, digital image subtraction, allows to see, as an example, the passage of contrast liquids in a tissue or in a venous or arterial duct; thanks to the possibility of looping, the acquired video can be reproduced several times to monitor more accurately the passage of the fluid within the area in question. Angiographic measurement is another useful function in the vascular field (QA Quantitative Angiography) that allows the measurement of stenoses. Finally, fluoroscopy allows the correct positioning of stents or expanders.
Neeo is used in various interventional and diagnostic procedures in traumatology and orthopedics wards and operating rooms as well. Thanks to low-dose fluoroscopy, it is possible to use the device for positioning bone or subcutaneous grafts, inserting K-wire (Kirschner wire) for stabilization of bone fragments or for the correct positioning of prostheses. The low dose emitted ensures safe use for both the patient and the surgeon or doctor on the operating field.
On the control panel there is a large touch screen display that allows to adjust the basic functions of the equipment. From this display it is possible to select and adjust the fluoroscopic data for the examination, activate or deactivate the laser pointer, select between pulsed, one shot or standard fluoroscopy, rotate the image and perform all operations on collimator. The four side buttons on the display offer the possibility to move the bow vertically thanks to an extremely silent motor.
Neeo has two 19 “medical grade monitors that can be positioned according to the needs of the medical practitioner. Work monitors and feedback monitors are separated to be managed independently. The possible movements are: rotation, revolution, tilting and possibility of height adjustment.
Features:
Click Here To Download Catalogue | Little Sister Table Top Autoclave: This sterilizer is a table type in safety and auto controlled,which is designed for the unit of medical health, pharmacy, science and technology and mostly suitable for sterilizing of surgical,dental and eye instruments, glassware medicine, culture medium and biological dressing, food and other goods.
Features:
| Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage.With this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||






Reviews
There are no reviews yet.