DynaMesh-IPST
$0.00
Shipped From Abroad
DynaMesh®-IPST, DynaMesh®-IPST visible, DynaMesh®-IPST-D visible, DynaMesh®-IPST-R and DynaMesh®-IPST-R visible implants are intended for the surgical treatment of parastomal hernias, and the prevention (not DynaMesh®-IPST-R or DynaMesh®-IPST-R visible) of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the stoma.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

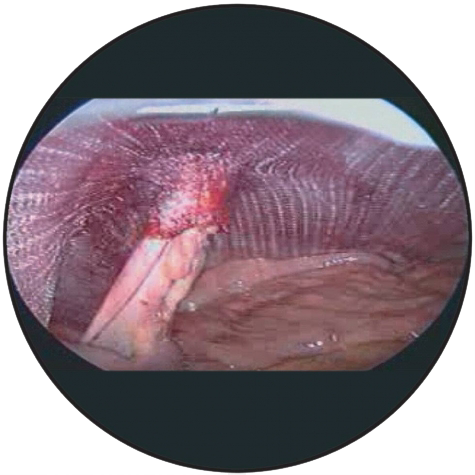
3D Funnel
The device has a passage point with a connected funnel for the passage of the intestine. The base area of the device must be positioned in contact with the tissue, as flat and smooth as possible, with the funnel pointing in the direction of the abdominal cavity.
When selecting the device, the diameter of the passage point should be chosen so that the intestine is tightly sheathed in the funnel.
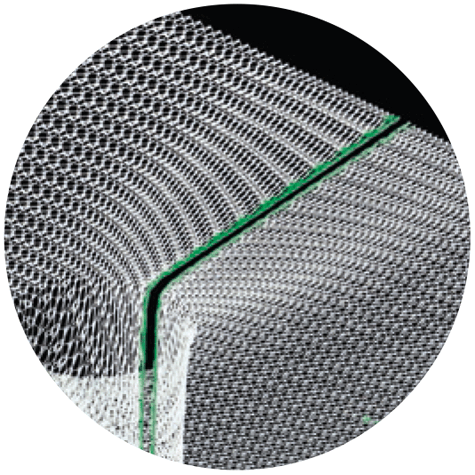
Prefabricated Slit & Smooth Warp-Knitted Selvedges
Only DynaMesh®-IPST-R and DynaMesh®-IPST-R visible have a one-sided slit along the funnel and the base area (mesh flaps), starting from the passage point.
During positioning, the tight sheathing of the intestine in the funnel is achieved through appropriate overlapping of the mesh flaps with non-absorbable closure of the aperture.
Specifications
| Product | DynaMesh®-IPST (1) DynaMesh®-IPST visible (2) DynaMesh®-IPST-D visible (3) DynaMesh®-IPST-R (4) DynaMesh®-IPST-R visible (5) |
| Surgical Treatment | Parastomal Hernia (Repair / Prevention) (1) – (3) / (Repair) (4) (5) |
| Surgical Approach | Minimally Invasive / Open |
| Surgical Technique | Chimney Technique |
| Mesh Position | Intraperitoneal |
| Fixation | Suture / Tacks / No Fibrin Glue |
| Green Filament Ends |
|
| Smooth Warp-Knitted Selvedges | |
| Visible Technology |
|
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 85% (w/w) (1) – (5) – Polypropylene (PP) (CAS 9003-07-0) < 13% (w/w) (1) – (5) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – (5) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) (3) (5) |
| Polymers (Monofilament) | PVDF, PP |
| Biocompatibility |
|
| Ageing Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR71B] |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
DynaMesh®-IPST implants (1)-(5) must be inserted via an appropriate minimally invasive or open approach and must be placed intraperitoneally.
DynaMesh®-IPST implants (1)-(5) have a parietal side and a visceral side. The parietal side is identified by green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
When using DynaMesh®-IPST-D visible (3), attention must be paid to the side specificity (left-sided/right-sided stoma), through which a particularly large overlap in a cranial as well as in a medial direction is ensured.
When positioning DynaMesh®-IPST-R and DynaMesh®-IPST-R visible (4) (5), the tight sheathing of the intestine in the funnel is achieved through appropriate overlapping of the mesh flaps with non-absorbable closure of the aperture
Quick Comparison
| DynaMesh-IPST remove | 18Ltrs Bucket Autoclave remove | Littmann Classic III Stethoscope remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | Bistos BT- 720 Patient Monitor remove | Spinal Epidural Pack remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-IPST remove | 18Ltrs Bucket Autoclave remove | Littmann Classic III Stethoscope remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | Bistos BT- 720 Patient Monitor remove | Spinal Epidural Pack remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  |  |  |  |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-6 | SF1033560084-7 | SF1033560084-34 | SF1033560084-37 | SF1033560059-8 | SF1033560084-25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
|
| $34.00 | $45.00 | $330.00 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-IPST, DynaMesh®-IPST visible, DynaMesh®-IPST-D visible, DynaMesh®-IPST-R and DynaMesh®-IPST-R visible implants are intended for the surgical treatment of parastomal hernias, and the prevention (not DynaMesh®-IPST-R or DynaMesh®-IPST-R visible) of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the stoma.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In Stock
| In Stock
The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
Omron M2 Upper Arm Blood Pressure Monitor Medium CufF: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage. zWith this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
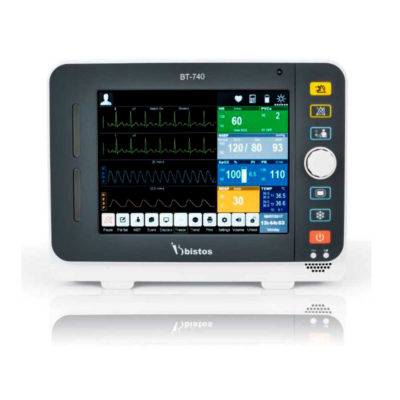
| Shipped from abroad Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer. Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
It is for performing spinal/epidural or combined spinal/epidural or never-block loco-regional anaesthesia on patinet in clinical surgery.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
3D Funnel
The device has a passage point with a connected funnel for the passage of the intestine. The base area of the device must be positioned in contact with the tissue, as flat and smooth as possible, with the funnel pointing in the direction of the abdominal cavity.
When selecting the device, the diameter of the passage point should be chosen so that the intestine is tightly sheathed in the funnel.
Prefabricated Slit & Smooth Warp-Knitted Selvedges Only DynaMesh®-IPST-R and DynaMesh®-IPST-R visible have a one-sided slit along the funnel and the base area (mesh flaps), starting from the passage point. During positioning, the tight sheathing of the intestine in the funnel is achieved through appropriate overlapping of the mesh flaps with non-absorbable closure of the aperture. Specifications
| Features:
| The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
Full adult and pediatric auscultation is available through its dual head chestpiece simply by rotating the central spline to alternate between the two sides. The more compact one piece diaphragm and rim assemblies have further increased acoustic sensitivity. The binaurals have been redesigned with larger diameter ear tubes and a thicker single lumen binaural to transfer sound more effectively. The selector spline is now recessed to allow a flush fit with the binaural tube and now features a visual indicator for easy identification of the operational diaphragm.
Features
| Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage.With this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer.
Features:
Click Here To Download Catalogue | It is for performing spinal/epidural or combined spinal/epidural or never-block loco-regional anaesthesia on patinet in clinical surgery.
Component:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||











Reviews
There are no reviews yet.