DynaMesh-SIS direct
$0.00
Shipped From Abroad
DynaMesh®-SIS direct, DynaMesh®-SIS direct soft, and DynaMesh®-SIS direct visible implants are intended to be used as a mid-urethral sling for the surgical treatment of stress urinary incontinence caused by a hypermobile urethra and/or intrinsic sphincter deficiency. The devices permanently reinforce the soft tissue of the pelvic floor.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

Retropubic Tape Position
(Inside-Out)

Transobturator Tape Position
(Outside-In or Inside-Out)

DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible are positioned using the inside-out technique in case of a retropubic tape position, and using the outside-in or inside-out technique in case of a transobturator tape position.
The devices have a thread at the ends of the tape that is attached to the surgical instrument.
Several reusable instruments are available separately to position the device:

DynaMesh®-ISR01:
Instrument for transvaginal access for retropubic positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the inside-out technique.

Diameter: 5-7 cm
DynaMesh®-IST01 / DynaMesh®-IST02 / DynaMesh®-IST03:
Instrument set consisting of two instruments for transvaginal access for transobturator positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the inside-out or outside-in technique.

DynaMesh®-IVT01:
Instrument for transvaginal access for transobturator positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the outside-in technique.
Specifications
| Product | DynaMesh®-SIS (1) DynaMesh®-SIS soft (2) DynaMesh®-SIS visible (3) |
| Surgical Treatment | Stress Urinary Incontinence (SUI) |
| Surgical Approach | Transvaginal |
| Surgical Technique | TVT – Retropubic – Inside-Out / TOT – Transobturator – Outside-In |
| Fixation | None |
| Smooth Warp-Knitted Selvedges |
|
| Shape Stability |
|
| Defined Elasticity |
|
| Visible Technology |
|
| Materials |
– Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) – (3)
– Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) (2) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (3) |
| Polymer (Monofilament) |
PVDF
|
| Biocompatibility |
|
| Ageing Resistance |
|
| Effective Porosity | |
| Klinge’s Mesh Classification | Class 1a [102P, TR11] |
Quick Comparison
| DynaMesh-SIS direct remove | Littmann Classic III Stethoscope remove | Bistos BT- 720 Patient Monitor remove | Bistos BT- 410 Medical Head Lamp remove | Bistos BT- 740 Patient Monitor remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-SIS direct remove | Littmann Classic III Stethoscope remove | Bistos BT- 720 Patient Monitor remove | Bistos BT- 410 Medical Head Lamp remove | Bistos BT- 740 Patient Monitor remove | Bistos BT-770-12.1" Touchscreen Patient Monitor remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image | 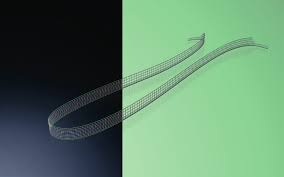 |  |  |  | 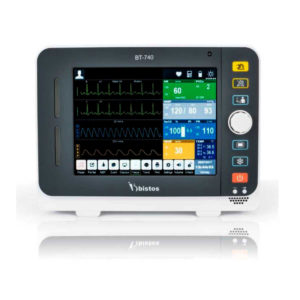 | 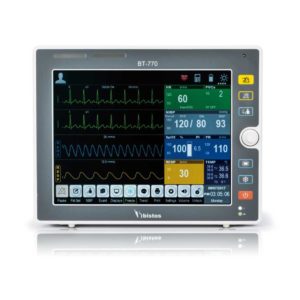 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-8 | SF1033560084-34 | SF1033560059-8 | SF1033560059-6 | SF1033560059-9 | SF1033560059-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $34.00 | $330.00 | $132.00 | $715.00 | $902.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-SIS direct, DynaMesh®-SIS direct soft, and DynaMesh®-SIS direct visible implants are intended to be used as a mid-urethral sling for the surgical treatment of stress urinary incontinence caused by a hypermobile urethra and/or intrinsic sphincter deficiency. The devices permanently reinforce the soft tissue of the pelvic floor.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In Stock
The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
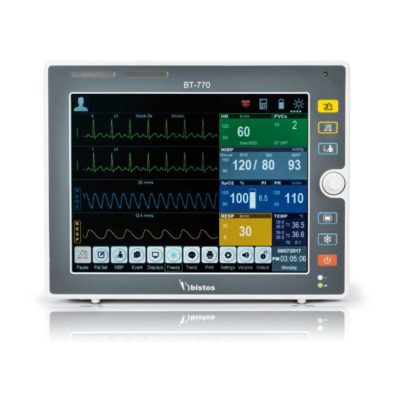
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from abroad Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer. Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from abroad Bistos BT- 410 Medical Head Lamp - Head-worn light BT-410 provides not only comfortable wear but also convenient for use during examinations or operations. - Ultra bright LED light with High density LED - Easy to adjust head strap & an angle - Extensive LED lifetime (more than 50,000 hours) - More than 4 hours of continuous use - An additional astral LED lamp (optional) - Attachable loupe (optional) Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from Abroad Bistos BT - 740 Patient Monitor: Bistos Patient Vital Signs Monitor, ECG, Resp., SpO2, NIBP, Temp., BT-740. The Bistos BT-740 is a multi-parameter patient monitor with an 8.4" TFT Color screen. The standard paramaters are ECG, respiratory, SpO2, NIBP and temperature. Additional options are EtCO2, IBP, Multi-gas, CO and Masimo SpO2. The BT-740 has a fanless design for quiet operation. Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | Shipped from Abroad The Bistos BT-770 patient monitor is equipped with a 12.1" touchscreen display, which allows for an easy operation and readability with a powerful rechargeable battery guaranteeing a continuous operation of 5 hours to monitor ECG, SpO2, NIBP, temperature and respiration Delivery & Availability: Typically 14 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
Retropubic Tape Position
(Inside-Out)
Transobturator Tape Position
(Outside-In or Inside-Out)
DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible are positioned using the inside-out technique in case of a retropubic tape position, and using the outside-in or inside-out technique in case of a transobturator tape position.
The devices have a thread at the ends of the tape that is attached to the surgical instrument.
Several reusable instruments are available separately to position the device:
DynaMesh®-ISR01:
Instrument for transvaginal access for retropubic positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the inside-out technique.
DynaMesh®-IST01 / DynaMesh®-IST02 / DynaMesh®-IST03:
Instrument set consisting of two instruments for transvaginal access for transobturator positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the inside-out or outside-in technique.
DynaMesh®-IVT01: Instrument for transvaginal access for transobturator positioning of DynaMesh®-SIS, DynaMesh®-SIS soft and DynaMesh®-SIS visible using the outside-in technique. Specifications
| The Classic III stethoscope brings new design, materials, and technology to the Littmann stethoscope series that’s been used and trusted by millions of medical professionals worldwide for decades. With a two-sided chestpiece, dual tunable diaphragms, improved tubing, and much more, the Classic III stethoscope combines the best of new and traditional.
Full adult and pediatric auscultation is available through its dual head chestpiece simply by rotating the central spline to alternate between the two sides. The more compact one piece diaphragm and rim assemblies have further increased acoustic sensitivity. The binaurals have been redesigned with larger diameter ear tubes and a thicker single lumen binaural to transfer sound more effectively. The selector spline is now recessed to allow a flush fit with the binaural tube and now features a visual indicator for easy identification of the operational diaphragm.
Features
| Bistos BT - 720 Patient Monitor: Bistos Portable Patient Vital Signs Monitor, SpO2, Pulse, *NIBP, BT-720. The Bistos BT-720 is a compact size vital signs monitor with the standard parameters of SpO2 and Pulse. You can also add the NIBP and/or Masimo SpO2 at an additional cost. The BT-720 has a 4.3" color touch screen The PC software allows the vital signs information to be transferred and analyzed on a computer.
Features:
Click Here To Download Catalogue | Bistos BT- 410 Medical Head Lamp - Head-worn light BT-410 provides not only comfortable wear but also convenient for use during examinations or operations. - Ultra bright LED light with High density LED - Easy to adjust head strap & an angle - Extensive LED lifetime (more than 50,000 hours) - More than 4 hours of continuous use - An additional astral LED lamp (optional) - Attachable loupe (optional).
Features:
Illumination Uniformity:
Click Here To Download Catalogue | Bistos BT - 740 Patient Monitor: Bistos Patient Vital Signs Monitor, ECG, Resp., SpO2, NIBP, Temp., BT-740. The Bistos BT-740 is a multi-parameter patient monitor with an 8.4" TFT Color screen. The standard paramaters are ECG, respiratory, SpO2, NIBP and temperature. Additional options are EtCO2, IBP, Multi-gas, CO and Masimo SpO2. The BT-740 has a fanless design for quiet operation.
Features:
Click Here To Download Catalogue |
Bistos BT-770 is a 12.1" touchscreen patient monitor designed for easy operations.
SPECIFICATIONS
Click Here To Download Catalogue | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||








Reviews
There are no reviews yet.