DynaMesh-IPST
$0.00
Shipped From Abroad
DynaMesh®-IPST, DynaMesh®-IPST visible, DynaMesh®-IPST-D visible, DynaMesh®-IPST-R and DynaMesh®-IPST-R visible implants are intended for the surgical treatment of parastomal hernias, and the prevention (not DynaMesh®-IPST-R or DynaMesh®-IPST-R visible) of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the stoma.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

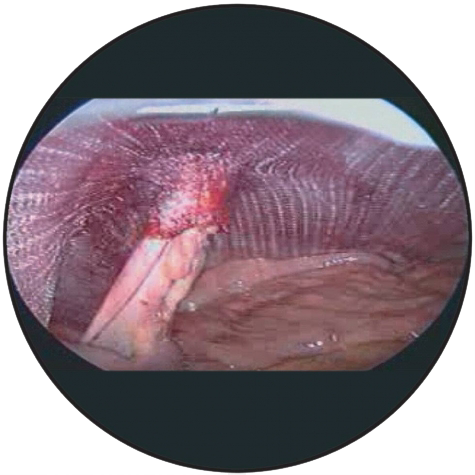
3D Funnel
The device has a passage point with a connected funnel for the passage of the intestine. The base area of the device must be positioned in contact with the tissue, as flat and smooth as possible, with the funnel pointing in the direction of the abdominal cavity.
When selecting the device, the diameter of the passage point should be chosen so that the intestine is tightly sheathed in the funnel.
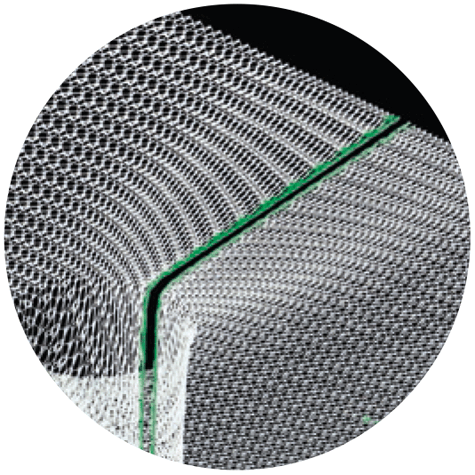
Prefabricated Slit & Smooth Warp-Knitted Selvedges
Only DynaMesh®-IPST-R and DynaMesh®-IPST-R visible have a one-sided slit along the funnel and the base area (mesh flaps), starting from the passage point.
During positioning, the tight sheathing of the intestine in the funnel is achieved through appropriate overlapping of the mesh flaps with non-absorbable closure of the aperture.
Specifications
| Product | DynaMesh®-IPST (1) DynaMesh®-IPST visible (2) DynaMesh®-IPST-D visible (3) DynaMesh®-IPST-R (4) DynaMesh®-IPST-R visible (5) |
| Surgical Treatment | Parastomal Hernia (Repair / Prevention) (1) – (3) / (Repair) (4) (5) |
| Surgical Approach | Minimally Invasive / Open |
| Surgical Technique | Chimney Technique |
| Mesh Position | Intraperitoneal |
| Fixation | Suture / Tacks / No Fibrin Glue |
| Green Filament Ends |
|
| Smooth Warp-Knitted Selvedges | |
| Visible Technology |
|
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 85% (w/w) (1) – (5) – Polypropylene (PP) (CAS 9003-07-0) < 13% (w/w) (1) – (5) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – (5) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) (3) (5) |
| Polymers (Monofilament) | PVDF, PP |
| Biocompatibility |
|
| Ageing Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR71B] |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
DynaMesh®-IPST implants (1)-(5) must be inserted via an appropriate minimally invasive or open approach and must be placed intraperitoneally.
DynaMesh®-IPST implants (1)-(5) have a parietal side and a visceral side. The parietal side is identified by green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
When using DynaMesh®-IPST-D visible (3), attention must be paid to the side specificity (left-sided/right-sided stoma), through which a particularly large overlap in a cranial as well as in a medial direction is ensured.
When positioning DynaMesh®-IPST-R and DynaMesh®-IPST-R visible (4) (5), the tight sheathing of the intestine in the funnel is achieved through appropriate overlapping of the mesh flaps with non-absorbable closure of the aperture
Quick Comparison
| DynaMesh-IPST remove | De Lineb Oxygen Face Mask remove | 18Ltrs Bucket Autoclave remove | Little Sister Table Top Autoclave remove | Bettermed BT667E Electric Operating Table remove | Ambu Oval Silicone resuscitator (Ambu Bag) remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-IPST remove | De Lineb Oxygen Face Mask remove | 18Ltrs Bucket Autoclave remove | Little Sister Table Top Autoclave remove | Bettermed BT667E Electric Operating Table remove | Ambu Oval Silicone resuscitator (Ambu Bag) remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  |  |  |  |  |  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-6 | SF1033560084-39 | SF1033560084-7 | SF1033560084-46 | SF1033560084-50 | SF1033560084-62 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
| $1.40 |
| $1,350.00 | $2,178.00 | $27.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | In stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | In stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-IPST, DynaMesh®-IPST visible, DynaMesh®-IPST-D visible, DynaMesh®-IPST-R and DynaMesh®-IPST-R visible implants are intended for the surgical treatment of parastomal hernias, and the prevention (not DynaMesh®-IPST-R or DynaMesh®-IPST-R visible) of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the stoma.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In stock
De Lineb Oxygen Face Mask: This mask provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask).
| In Stock
| Out stock Little Sister Table Top Autoclave: This sterilizer is a table type in safety and auto controlled,which is designed for the unit of medical health, pharmacy, science and technology and mostly suitable for sterilizing of surgical,dental and eye instruments, glassware medicine, culture medium and biological dressing, food and other goods. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
This operation table is used for the surgeries of chest ,abdomen ,ophthalmology,obstetrics,and urology.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock The Ambu Oval Silicone resuscitator is designed for manual ventilation of neonates though to adults. Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
3D Funnel
The device has a passage point with a connected funnel for the passage of the intestine. The base area of the device must be positioned in contact with the tissue, as flat and smooth as possible, with the funnel pointing in the direction of the abdominal cavity.
When selecting the device, the diameter of the passage point should be chosen so that the intestine is tightly sheathed in the funnel.
Prefabricated Slit & Smooth Warp-Knitted Selvedges Only DynaMesh®-IPST-R and DynaMesh®-IPST-R visible have a one-sided slit along the funnel and the base area (mesh flaps), starting from the passage point. During positioning, the tight sheathing of the intestine in the funnel is achieved through appropriate overlapping of the mesh flaps with non-absorbable closure of the aperture. Specifications
| De Lineb Oxygen Face Mask: This mask provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask).
Features:
| Features:
| Little Sister Table Top Autoclave: This sterilizer is a table type in safety and auto controlled,which is designed for the unit of medical health, pharmacy, science and technology and mostly suitable for sterilizing of surgical,dental and eye instruments, glassware medicine, culture medium and biological dressing, food and other goods.
Features:
| This operation table is used for the surgeries of chest, abdomen, ophthalmology, obstetricsand urology, etc.It has the following advantages:
●The board can shift longitudinally. It is made of Transparent material material.it can be X-rayed or scan when using along with C-ARM .
●The board is controlled by electric handspike to move up and down, lean from left to right and move back and forth.
●The leg board is dismantable. It is manual rotation and could be ooutreached with the force from the spring and gravity, convenient for urology surgery.
Technical Specification
| The Ambu Oval Silicone resuscitator is designed for manual ventilation of neonates though to adults.
Features
* International guidelines recommend that all manual resuscitators for infant and pediatric patients should be equipped with a pressure limitation mechanism so the airway pressure does not exceed 45 cm H2O. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||









Reviews
There are no reviews yet.