DynaMesh-IPST
$0.00
Shipped From Abroad
DynaMesh®-IPST, DynaMesh®-IPST visible, DynaMesh®-IPST-D visible, DynaMesh®-IPST-R and DynaMesh®-IPST-R visible implants are intended for the surgical treatment of parastomal hernias, and the prevention (not DynaMesh®-IPST-R or DynaMesh®-IPST-R visible) of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the stoma.
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
Description

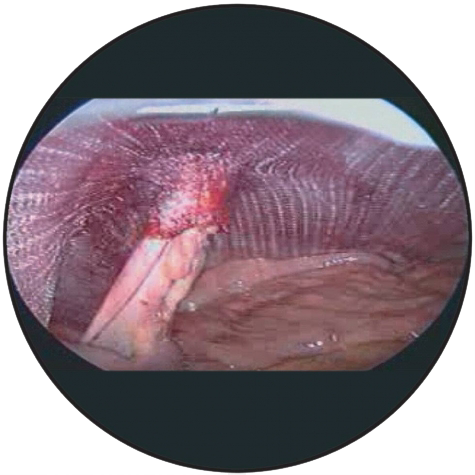
3D Funnel
The device has a passage point with a connected funnel for the passage of the intestine. The base area of the device must be positioned in contact with the tissue, as flat and smooth as possible, with the funnel pointing in the direction of the abdominal cavity.
When selecting the device, the diameter of the passage point should be chosen so that the intestine is tightly sheathed in the funnel.
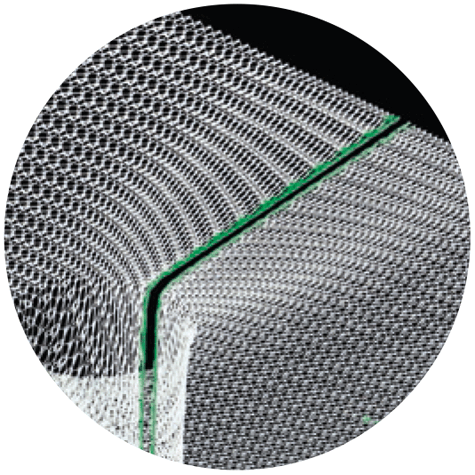
Prefabricated Slit & Smooth Warp-Knitted Selvedges
Only DynaMesh®-IPST-R and DynaMesh®-IPST-R visible have a one-sided slit along the funnel and the base area (mesh flaps), starting from the passage point.
During positioning, the tight sheathing of the intestine in the funnel is achieved through appropriate overlapping of the mesh flaps with non-absorbable closure of the aperture.
Specifications
| Product | DynaMesh®-IPST (1) DynaMesh®-IPST visible (2) DynaMesh®-IPST-D visible (3) DynaMesh®-IPST-R (4) DynaMesh®-IPST-R visible (5) |
| Surgical Treatment | Parastomal Hernia (Repair / Prevention) (1) – (3) / (Repair) (4) (5) |
| Surgical Approach | Minimally Invasive / Open |
| Surgical Technique | Chimney Technique |
| Mesh Position | Intraperitoneal |
| Fixation | Suture / Tacks / No Fibrin Glue |
| Green Filament Ends |
|
| Smooth Warp-Knitted Selvedges | |
| Visible Technology |
|
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 85% (w/w) (1) – (5) – Polypropylene (PP) (CAS 9003-07-0) < 13% (w/w) (1) – (5) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – (5) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) (3) (5) |
| Polymers (Monofilament) | PVDF, PP |
| Biocompatibility |
|
| Ageing Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR71B] |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
DynaMesh®-IPST implants (1)-(5) must be inserted via an appropriate minimally invasive or open approach and must be placed intraperitoneally.
DynaMesh®-IPST implants (1)-(5) have a parietal side and a visceral side. The parietal side is identified by green filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.
When using DynaMesh®-IPST-D visible (3), attention must be paid to the side specificity (left-sided/right-sided stoma), through which a particularly large overlap in a cranial as well as in a medial direction is ensured.
When positioning DynaMesh®-IPST-R and DynaMesh®-IPST-R visible (4) (5), the tight sheathing of the intestine in the funnel is achieved through appropriate overlapping of the mesh flaps with non-absorbable closure of the aperture
Quick Comparison
| DynaMesh-IPST remove | Spinal Epidural Pack remove | McKesson Sprague Rappaport Stethoscope remove | Littman Stethoscope remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | Bistos BT- 740 Patient Monitor remove | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | DynaMesh-IPST remove | Spinal Epidural Pack remove | McKesson Sprague Rappaport Stethoscope remove | Littman Stethoscope remove | Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff remove | Bistos BT- 740 Patient Monitor remove | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Image |  |  |  |  |  |  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SKU | SF1033560130161-6 | SF1033560084-25 | SF1033560084-35 | SF1033560084-5 | SF1033560084-37 | SF1033560059-9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rating | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Price |
|
| $17.00 | $13.20 | $45.00 | $715.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Availability | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Add to cart | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Shipped From Abroad
DynaMesh®-IPST, DynaMesh®-IPST visible, DynaMesh®-IPST-D visible, DynaMesh®-IPST-R and DynaMesh®-IPST-R visible implants are intended for the surgical treatment of parastomal hernias, and the prevention (not DynaMesh®-IPST-R or DynaMesh®-IPST-R visible) of parastomal hernias following ostomy surgery, and permanently bridge and reinforce the soft tissue of the abdominal wall in the area of the stoma.
Delivery & Availability:
Typically 10-21 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information.
| In Stock
It is for performing spinal/epidural or combined spinal/epidural or never-block loco-regional anaesthesia on patinet in clinical surgery.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
McKesson Sprague Rappaport Stethoscope: The McKesson Brand Two Sided Classic Nurse Stethoscope is an invaluable Diagnostic Instrument used by medical professionals when diagnosing heart, lung and vascular conditions. This high-quality stethoscope features an adjustable aluminum binaural, a single lumen 22-inch tube, a two-sided wide frequency chest piece, and comfortable ear tips.
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In Stock
Delivery & Availability: Typically 2 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | In stock
Omron M2 Upper Arm Blood Pressure Monitor Medium CufF: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage. zWith this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| Shipped from Abroad Bistos BT - 740 Patient Monitor: Bistos Patient Vital Signs Monitor, ECG, Resp., SpO2, NIBP, Temp., BT-740. The Bistos BT-740 is a multi-parameter patient monitor with an 8.4" TFT Color screen. The standard paramaters are ECG, respiratory, SpO2, NIBP and temperature. Additional options are EtCO2, IBP, Multi-gas, CO and Masimo SpO2. The BT-740 has a fanless design for quiet operation. Delivery & Availability: Typically 7 working days – excluding furniture and heavy/bulky equipment. Please contact us for further information. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Content |
3D Funnel
The device has a passage point with a connected funnel for the passage of the intestine. The base area of the device must be positioned in contact with the tissue, as flat and smooth as possible, with the funnel pointing in the direction of the abdominal cavity.
When selecting the device, the diameter of the passage point should be chosen so that the intestine is tightly sheathed in the funnel.
Prefabricated Slit & Smooth Warp-Knitted Selvedges Only DynaMesh®-IPST-R and DynaMesh®-IPST-R visible have a one-sided slit along the funnel and the base area (mesh flaps), starting from the passage point. During positioning, the tight sheathing of the intestine in the funnel is achieved through appropriate overlapping of the mesh flaps with non-absorbable closure of the aperture. Specifications
| It is for performing spinal/epidural or combined spinal/epidural or never-block loco-regional anaesthesia on patinet in clinical surgery.
Component:
| McKesson Sprague Rappaport Stethoscope: The McKesson Brand Two Sided Classic Nurse Stethoscope is an invaluable Diagnostic Instrument used by medical professionals when diagnosing heart, lung and vascular conditions. This high-quality stethoscope features an adjustable aluminum binaural, a single lumen 22-inch tube, a two-sided wide frequency chest piece, and comfortable ear tips.
Features
| Littman Stethoscope Features:
| Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff: Omron M2 Upper Arm Blood Pressure Monitor Medium Cuff is a useful tool for clinical management of patients with hypertension. The ease with which using this machine can be learned is a major advantage.With this device, you can regularly check your blood pressure level and even get the same consistent accuracy as in your doctor’s office. This digital BP monitor has an arm cuff and a built-in LCD screen to display of the blood pressure readings. A wonderful feature that comes with this monitor is that readings given by the monitor can be stored in the monitor’s memory and can be printed out later.
| Bistos BT - 740 Patient Monitor: Bistos Patient Vital Signs Monitor, ECG, Resp., SpO2, NIBP, Temp., BT-740. The Bistos BT-740 is a multi-parameter patient monitor with an 8.4" TFT Color screen. The standard paramaters are ECG, respiratory, SpO2, NIBP and temperature. Additional options are EtCO2, IBP, Multi-gas, CO and Masimo SpO2. The BT-740 has a fanless design for quiet operation.
Features:
Click Here To Download Catalogue | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dimensions | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||










Reviews
There are no reviews yet.